Determination of Phenolic Compounds in Mainstream Tobacco Smoke
Health Canada
T-114 December 31, 1999
Table of Contents
- Scope of Applications
- Normative references
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- Modifications for Intensive Smoking
- References
- Appendices
1 Scope of Applications
- This method describes the extraction and determination of phenolic compounds in the total particulate matter (TPM) of mainstream tobacco smoke by reversed phase high performance liquid chromatography (HPLC).
- Applicable to the trapping and quantitation of phenolic compounds on glass fiber filter discs (pads) in mainstream tobacco smoke.
2 Normative References
- American Society for Testing and Materials (ASTM) D 1193-77 - Standard Specification for Reagent Water, Version 1977.
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Smoke (for MS and SS methods), 1999-12-31.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- Five cigarettes* per port are smoked on a standard 20 port linear smoking machine.
*For other tobacco products, select a number such that breakthrough does not occur. - The mainstream total particulate matter (TPM) is collected on the pads. The pad is then extracted with 40 mL of 1 % acetic acid (HoAC).
- An aliquot of the TPM extract is syringe filtered, diluted and subjected to reversed-phase gradient liquid chromatography.
- Phenols are monitored using selective fluorescence detection and quantified by comparison to an external standard calibration.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- Analytical Balance capable of measuring to at least four decimal places.
- Wrist Action Shaker.
- Glassware Drying Oven.
- Tweezers and gloves for transferring pads.
- 150 mL Erlenmeyer flasks with ground glass stoppers.
- PC controlled High Performance Liquid Chromatography System consisting of:
- Solvent Delivery System - tertiary gradient pump.
- Refrigerated Autosampler with 20 µL sampling loop.
- Spectrofluorometer.
- Column Temperature Modifier.
- Cooling Bath.
- Work Station.
- RP 18 e 5 µm, 250 mm × 4 mm Chromatographic HPLC Column.
- Disposable Guard Column 10 mm × 4 mm.
- Volumetric flasks - 10 mL, 25 mL, 50 mL, Actinic Red.
- Glass Micropipettes - assorted volumes (100, 150, 300, 400, 500, 800, 1000, and 2000 µL).
- Glass Transfer Pipettes - 1, 2, 5, 6, 7, 8, and 20 mL.
- Erlenmeyer flasks with ground glass joints - 50 mL, Actinic Red.
- Glass Graduated Measuring Cylinder - 25 mL, 50 mL.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade in quality.
- Syringe Filter 0.45 µm PVDF.
- Disposable syringes.
- Disposable Glass Pasteur Pipettes.
- Disposable gloves.
- Rubber Bulbs.
- Autosampler vials, screw caps and septa.
- Masking Tape.
- Methanol - Distilled in Glass (DIG).
- Acetonitrile - DIG.
- Isopropanol - DIG.
- Acetic Acid - HPLC grade.
- Type I water as specified in ASTM D 1193.
- Hydroquinone > 99 % purity.
- Resorcinol > 99 % purity.
- Catechol > 99 % purity.
- Phenol > 99 % purity.
- m-Cresol > 99 % purity.
- p-Cresol > 99 % purity.
- o-Cresol > 99 % purity.
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
8 Preparation of Solutions
- Prepare 4 L fresh 1 % acetic acid solution in Type I water (40 mL HoAc diluted up to 4 L) and test by HPLC for contamination.
9 Preparation of Standards
- Primary (1°) Phenol Standards (See Appendix 1)
- Weigh approximately 25 mg of the following phenols (Hydroquinone, Resorcinol, Catechol, Phenol, m-Cresol, p-Cresol and o-Cresol) into individual 25 mL volumetric flasks and make up to the mark with fresh 1 % acetic acid solution.
- Concentrations will be approximately 1.0 mg/mL. Prepare fresh primary phenol stock standards every 10 working days.
- Secondary (2°) Phenol Standards (See Appendix 1)
- Take appropriate volumes of the Primary Phenol Standards and dilute to 10 mL with 1 % acetic acid.
- Prepare Secondary phenol standards fresh with each new primary stock standard.
- Tertiary (3°) Phenol Solution (See Appendix 1)
- Take corresponding volumes of each phenol solution and add to a single 50 mL flask. Dilute up to the mark with 1 % acetic acid.
- Prepare tertiary phenol solution fresh every five working days.
- Phenol Working Standards
- Take appropriate volumes (0.100 to 7.5 mL) of the tertiary phenol solution and dilute to 10 mL with 1 % acetic acid. (See Appendix 1)
- Transfer to autosampler vials.
- Phenol working standards are prepared fresh every five working days.
- Phenol Spiking Solution
- Add selected volumes of the phenol standards in a 50 mL volumetric flask and make up to the mark with 1 % acetic acid. (See Appendix 1)
- Prepare phenol spiking solution fresh every five working days.
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars shall be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115.
13 Sample Generation
- The TPM from the mainstream tobacco smoke from five cigarettes is collected per pad according to T-115.
- After all five test cigarettes per port are finished smoking:
- Take an additional three clearing puffs after the final cigarette.
- Record the weight of the glass fiber filter holder plus pad after smoking to determine TPM.
14 Sample Analysis
- Extraction of the Pad
- One run consists of 20 samples (pads). Process 20 samples at a time. Do not smoke more than can be analyzed in a 24-hour period. Hydroquinone is especially temperature and time sensitive.
- Remove the filter pad from the filter holder, fold it into quarters and wipe the inside of the holder with clean side of the pad and then insert into an appropriately labeled 50 mL actinic red Erlenmeyer flask using a clean pair of tweezers.
- Add 40 mL of 1 % HoAc to the flask containing the filter pad, stopper the flask and put a piece of 1" masking tape over the ground glass stopper to hold in place.
- Prepare a laboratory reagent blank (LRB) and laboratory fortified blank (LFB) with each smoking run as follows to demonstrate that interference from the analytical system, glassware, and reagents are not present.
LRB: Add one conditioned unused filter pad to a clean Erlenmeyer flask, add 40 mL of 1 % acetic acid solution and stopper the flask.
LFB: Add one conditioned unused filter pad to a clean Erlenmeyer flask, add 39 mL of 1 % acetic acid solution plus 1 mL of the phenol spiking solution and stopper the flask. - The flasks are then loaded and clamped onto a wrist-action shaker and agitated for 30 minutes. (The pad will be disintegrated).
- After shaking, dilute the acetic acid / TPM in 10 mL volumetric flasks and extract with 1 % acetic acid according the following schedule.
The following table provides information regarding the TPM per pad, volume of smoke extract and final volume required to achieve a particular dilution depending on the cigarette brand: light, regular or heavy.
Cigarette Brand TPM per pad (mg) Volume (mL) Smoke Extract * Final Vol (mL) Light 5 -15 0 No Dilution Regular 15 - 60 4 10 Heavy 60 - 100 2 10
*"Ultra Light" brands - Attach a 0.45 µm filter to a 5 cc disposable syringe and filter the smoke extract directly into autosampler vials in duplicate. Discard the first 10 drops, add a few drops to the vials and discard rinse, then fill to minimize head space.
*Regular and "Full Flavour" brands - Attach a 0.45 µm filter to a 5 cc disposable syringe and filter the smoke extract directly into a 10 mL volumetric flask that has been preloaded with 7 mL of 1 % acetic acid or as necessary to dilute the smoke extract to 10 mL. Mix the volumetric flask well and then, using a Pasteur pipette, fill autosampler vials in duplicate. Rinse vial first with filtered extract and then fill to minimize headspace. - The LRB and LFB are syringe filtered directly into autosampler vials.
- Attach a 0.45 µm filter to a 5 cc disposable syringe and filter the blank pad extract directly into autosampler vials in duplicate. Discard the first 10 drops; add a few drops to the vials and discard rinse; then fill to minimize head space
- Prepare a laboratory fortified matrix (LFM) using a standard control brand with each run of smoked samples.
The following table displays the laboratory fortified matrix prepared using a standard control brand, with each run of smoke samples.
Reagent sample LFM 1 % Acetic Acid (mL) 7 6 Phenol Spike (mL) 0 1 Smoke Extract (mL) 3 3 Total Volume (mL) 10 10
LFM Samples - Attach a 0.45 µm filter to a 5 cc disposable syringe and filter the smoke extract directly into a 10 mL volumetric flask that has been preloaded with 5 mL 1 % acetic acid plus 1 mL of the phenol spike. - Mix the volumetric flask well and then using a Pasteur pipette, fill autosampler vials in duplicate. Rinse vial first and then fill to minimize headspace.
- Place vials in a vial file and store in the refrigerator at 4 °C protected from light until analyzed.
- A run log is then generated to record the total time samples are at room temperature from smoking to the end of analysis.
Note: It is very critical that sample analysis be completed in minimal time without interruption, as the samples will decompose with prolonged exposure at room temperature.
- Instrument Analysis: HPLC Equipment
- High Performance Liquid Chromatography System consisting of:
Solvent Delivery System - tertiary gradient pump.
Refrigerated Autosampler with 20 µL sampling loop.
Programmable Wavelength Spectrofluorometer , set to Gain 100, ATTN 8.
Slit Width: Ex 18 nm, Em 18 nm. - Wavelength Profile
The following table displays the observed excitation and emission wavelengths at various times.
Time Initial Excitation (nm) Emission (nm) 0.0 304 338 5.5 274 298 32.0 274 298 33.0 304 338 - Cooling Bath with column temperature modifier attachment.
- Work Station.
- High Performance Liquid Chromatography System consisting of:
- Chromatographic Conditions (Reversed Phase Analysis)
- Column Temperature: 20 °C.
- Mobile Phase: Reagents
Solvent A: Prepare 2 L of 1 % Acetonitrile, 1 % Acetic Acid, 1 % IPA filter and degas. (UHP Helium sparged).
Solvent B: Prepare 2 L of 28 % Acetonitrile, 1 % Acetic Acid, 1 % IPA filter and degas. (UHP Helium sparged).
Solvent C: Acetonitrile BDH Omnisolve, (UHP Helium sparged). - Sample Wash: Solvent A.
- Mobile Phase: Gradient
Flowrate 1.5 mL/minute
The following table displays the percent composition of the solvents in the mobile phase for gradient run at various time (from 0 to 34 minutes), used for analyte separation during high performance liquid chromatography.
Time (minutes) Composition % A % B % C 0.0 100 0 0 5.0 100 0 0 15.0 75 25 0 20.0 25 75 0 28.0 0 100 0 30.0 0 0 100 32.0 0 0 100 34.0 95 0 5 Method End Action 100 0 0 (Equilibrate 10 minutes) - Sample vials are loaded onto the autosampler such that every 10th vial is a standard solution and in such quantities that the total analysis time does not exceed 24 hours.
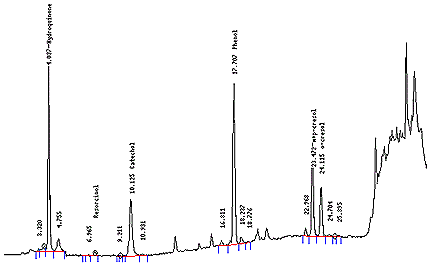
- Twenty µL of each sample vial is injected onto the HPLC. Elution pattern should be similar to Figure 2.
- Calculations
- Construct a Calibration Curve:
- Twenty µL of each calibration standard are injected onto the HPLC column and analyzed. Do in duplicate. Elution pattern should be similar to Figure 1.
- A calibration curve of the various hydroxybenzene compounds is prepared by plotting the concentration of the standards versus their respective peak areas.
- Determine the Response Factor.
- Sample Quantification
- The amount of the various phenolic compounds in smoke samples is quantified by the external standard method.
- The identification of peaks is by comparison of retention times with standards, and the spiking of smoke samples.
- Determination of Phenol Deliveries in [µg/cigarette]
Hydroxybenzene [µg/cig] = [Peak Area/ Resp. Factor] × [DF / # of Cigarettes]
where DF is the dilution factor.
- Construct a Calibration Curve:
15 Quality Control
- Typical Chromatograms
- See Figure 1, 2.
- Recoveries and Levels of Contamination
- Each analytical run of test cigarettes should also include:
- A Laboratory Reagent Blank (LRB) to evaluate the extent of any interference due to glassware, trapping reagents, filter pads, and analyzer effects.
- A Laboratory Fortified Blank (LFB) to evaluate the extent of potential analyte loss.
- A Laboratory Fortified Matrix (LFM) to assess matrix interference. This is accomplished by spiking a true sample with a known concentration and determining a per cent recovery.
- Each analytical run of test cigarettes should also include:
- Method Detection Limit (MDL) and Limit of Quantitation
- Method Detection Limit (MDL)
The method detection limit is determined by analyzing the lowest level standard at least 10 times as an unknown over several days. The MDL is then calculated as three times the standard deviation of these determinations. - Limit of Quantitation (LOQ)
The limit of quantification is determined by analyzing the lowest level standard at least 10 times as an unknown over several days. The LOQ is then calculated as 10 times the standard deviation of these determinations.
- Method Detection Limit (MDL)
- Stability of Reagents and Supplies
- All primary Phenol standards are prepared fresh weekly.
- All work standards and reagents are prepared fresh weekly.
- All samples are analyzed as soon as they are smoked and within 24 hours.
16 Modifications for Intense Smoking Conditions
- Under intense smoking conditions the number of cigarettes smoked is two.
17 Reference
- Risner, C.H. and Cash, S.L. A High Performance Liquid Chromatographic Determination of Major Phenolic Compounds in Tobacco Smoke, Journal of Chromatographic Science, 28, 1990.
Appendices
Appendix 1: Phenol Calibration Standards
(a): Phenol Standards
This table displays the phenol standards for various analytes. The table includes weight, purity and volume of the primary standards; as well as the volume, final volume and concentration of the secondary and tertiary standard solutions. The phenol analytes include hydroquinone, resorcinol, catechol, phenol, m-cresol, p-cresol, o-cresol, m+p-cresol.
| Phenol | Primary (1°) Standard * | Secondary (2°) Standard * | Tertiary (3°) Solution ** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight (g) | Purity (%) | Volume (mL) | Stock [mg/mL] | Vol (mL) 1 Stock | Dilute to Vol (mL) | Stock [mg/mL] | Vol (mL) Stock | Dilute to Vol (mL) | Stock [mg/mL] | |
| Hydroquinone | 0.023 | 99.0 | 25.0 | 0.9108 | 0.50 | 50.0 | 9.10800 | |||
| Resorcinol | 0.0232 | 99.0 | 25.0 | 0.91872 | 2.0 | 10.0 | 0.18374 | 0.20 | 50.0 | 0.73498 |
| Catechol | 0.0227 | 99.0 | 25.0 | 0.89892 | 0.25 | 50.0 | 4.49460 | |||
| Phenol | 0.0235 | 99.0 | 25.0 | 0.9306 | 0.50 | 50.0 | 9.30600 | |||
| m-Cresol | 0.0315 | 99.0 | 25.0 | 1.2474 | 1.0 | 10.0 | 0.12474 | 1.00 | 50.0 | 2.49480 |
| p-Cresol | 0.0225 | 99.0 | 25.0 | 0.891 | 0.5 | 10.0 | 0.04455 | 1.00 | 50.0 | 0.89100 |
| o-cresol | 0.0307 | 99.0 | 25.0 | 1.21572 | 0.4 | 10.0 | 0.04863 | 2.00 | 50.0 | 1.94515 |
| m+p-Cresol | 99.0 | 25.0 | 3.38580 | |||||||
* In 1 % (v/v) Acetic Acid
** In 1 % (v/v) Acetic Acid in a single 50 mL volumetric flask
(b): Phenol Working Standards
This table displays the concentrations of the working standards of various phenol analytes. These include hydroquinone, resorcinol, catechol, phenol, m-cresol, o-cresol, and m+p-cresol.
| Label | 5 | 10 | 100 | 200 | 350 | 500 | 750 | 1000 |
|---|---|---|---|---|---|---|---|---|
| Vol (mL) 3° | 0.050 | 0.100 | 1.000 | 2.000 | 3.500 | 5.000 | 7.500 | 10.000 |
| Phenol | [µg/mL] | [µg/mL] | [µg/mL] | [µg/mL] | [µg/mL] | [µg/mL] | [µg/mL] | [µg/mL] |
| Hydroquinone | 0.04554 | 0.09108 | 0.91080 | 1.82160 | 3.18780 | 4.55400 | 6.83100 | 9.10800 |
| Resorcinol | 0.00367 | 0.00735 | 0.07350 | 0.14700 | 0.25724 | 0.36749 | 0.55123 | 0.73498 |
| Catechol | 0.02247 | 0.04495 | 0.44946 | 0.89892 | 1.57311 | 2.24730 | 3.37095 | 4.49460 |
| Phenol | 0.04653 | 0.09306 | 0.93060 | 1.86120 | 3.25710 | 4.65300 | 6.97950 | 9.30600 |
| m-Cresol | 0.01247 | 0.02495 | 0.24948 | 0.49896 | 0.87318 | 1.24740 | 1.87110 | 2.49480 |
| p-Cresol | 0.00446 | 0.00891 | 0.08910 | 0.17820 | 0.31185 | 0.44550 | 0.66825 | 0.89100 |
| o-cresol | 0.00973 | 0.01945 | 0.19452 | 0.38903 | 0.68080 | 0.97258 | 1.45886 | 1.94515 |
| m+p-Cresol | 0.01693 | 0.03386 | 0.33858 | 0.67716 | 1.18503 | 1.69290 | 2.53935 | 3.38580 |
+ In 1 % (v/v) Acetic Acid in single 10 mL volumetric flasks
(c): Spiking Solution
This table contains the dilution information regarding primary and secondary stocks used to prepare spiking solution. Hydroquinone and phenol primary stocks were used, whereas o-cresol secondary stock was used in preparation of spiking solutions.
| Phenol | LFB Spiking Solution *** | LFM Spike ++ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stock Level | Stock [mg/mL] | Volume (mL) | Dilute Vol (mL) | Spike [µg/mL] | Analyzed [µg/mL] | Spike Vol (mL) | Dilute to Vol (mL) | Spike [µg/mL] | Analyzed [µg/mL] | |
| Hydroquinone | Primary | 0.9108 | 1.0 | 36.432 | 1.82160 | 36.432 | 3.64320 | |||
| Phenol | Primary | 0.9306 | 0.6 | 25.30 | 22.3344 | 1.11672 | 22.3344 | 2.23344 | ||
| o-cresol | Secondary | 0.04863 | 1.4 | 2.72321 | 0.13616 | 2.72321 | 0.27232 | |||
*** In 1% (v/v) Acetic Acid in a single 25 mL volumetric flask
++ In 1% (v/v) Acetic Acid in a single 10 mL volumetric flask
Figure 1: Chromatogram of a Typical Phenol Calibration Standard
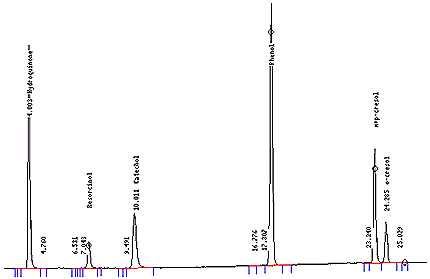
Figure 2: Chromatogram of the Analysis of TPM from Mainstream Tobacco Smoke for Phenols