Determination of Toxic Trace Metals in Sidestream Smoke
Health Canada
T–207, December 31, 1999
Table of Contents
- Scope of Applications
- Normative References
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Atomic Absorption Analysis
- Quality Control
- Modifications For Intensive Smoking
- References
- Appendices
1 Scope of Applications
- This method is applicable to the determination of nickel (Ni), lead (Pb), cadmium (Cd), chromium (Cr), arsenic (As), and selenium (Se) in sidestream tobacco smoke by Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Argon Plasma - Atomic Emission Spectroscopy (ICP-AES). The method is designed to quantitate these toxic trace metals in both the particulate phase and gaseous phase of sidestream smoke from cigarettes, cigarette equivalents, kreteks, bidis and cigars smoked on a linear smoking machine.
- Sidestream smoke is, effectively, all the smoke emitted from a cigarette other than the mainstream smoke. This is collected using a glass fishtail chimney assembly to direct the smoke to various trapping devices.
- Particulate phase metals are determined as those metals that become part of the sidestream smoke particulate matter, trapped on a glass fibre filter disc (pad), as well as metals bound to the particulate matter remaining on the glass wall of the fishtail chimney assembly.
- Gaseous phase metals are determined as those metals that may have reacted to form a gaseous species or particulate matter that is not retained in the normal Total Particulate Matter (TPM) condensate.
2 Normative References
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
- American Society for Testing and Materials (ASTM) D1193-77 - Standard Specifications for Reagent Water, Version 1977.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- Equidistant ports of a standard linear smoking machine are reconfigured with the British American Tobacco (BAT) fishtail chambers and flow-controlled vacuum pumps. Four cigarettes* per port are smoked beneath the fishtail chambers as specified by T-115. The smoke is swept up the chimney at the rate of 3 L/minute.
*For other tobacco products, select a number such that breakthrough does not occur. - The TPM of the sidestream smoke is collected on a 44 mm pad at the top of the chimney. The particulate matter trapped on the wall of the fishtail is washed off using the solution from the impingers (20 mL) followed by 10 mL of hydrogen peroxide plus 10 mL of fresh impinger solution. This rinse is added to a PMP Erlenmeyer flask with screw top closure. The pad is also added to the flask where it is extracted on a wrist action shaker. After 30 minutes of shaking, the mixture is transferred to a microwave digestion vessel by pouring it through a funnel with a plug of glass wool. The Erlenmeyer is then rinsed with an additional 2 X 10 mL of fresh impinger solution. These solutions are also added to the digestion vessel.
Note: Tar is extracted from the pad, rather than attempting to digest the entire pad, in order to reduce the amount of background metals from the pad. The magnitude of the variability in the pad from its digestion could be substantial in comparison to the amount of metals to be analyzed. - The gaseous phase metals are trapped by placing two impingers of a 10 % v/v nitric acid solution between the pad and the vacuum pump. Both impinger solutions are added to the same digestion vessel and subjected to microwave digestion.
- When the digestion is complete, the vessels are removed from the digestor, allowed to cool, and transferred to a volumetric flask where they are made to volume with Type I water.
- The digestates are then analyzed by flameless atomic absorption spectroscopy (or graphite furnace atomic absorption). This method uses pyrolytic coated partition tubes for increased resistivity toward acid therefore increasing the lifetime of the tube and sensitivity to the analyte.
- Quantitation is achieved by interpolating the relevant calibration curves prepared from standard metal solutions of aqueous standards in the same acid concentration to minimize matrix affects. For some metals the use of a matrix modifier is required to prevent loss of analyte during the analysis.
Note: Arsenic and selenium may also be analyzed by hydride generation using sodium borohydride. Extreme care, and a secondary digestion procedure, must be used to ensure these metals are in the proper oxidation state for the hydride reaction to quantitatively occur. This also requires the digestate to be further diluted resulting in a loss in sensitivity.
Note: The analysis of Cd, Pb, Ni, and Cr, can also be analyzed by ICP-AES in conjunction with an ultrasonic nebulizer in order to increase sensitivity.
Important: The cleaning of glassware and the cleanliness of the environment in which the analysis is performed, has a direct effect on the accuracy and precision of the method. In order to achieve accurate results, all glassware must be cleaned immediately prior to use with dilute HCl (1+1) and then rinsed with Type I water.
Note: The testing and evaluation of certain products against this method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this standard has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- 70 mL impingers without frit.
- 1/4" ID x 3/8" OD ester grade Tygon tubing.
- 1/4" Nalgene connectors.
- Vacuum Pumps.
- Flow meters.
- Fishtail Chambers - BAT.
- 125 mL Polymethylpentene (PMP) Erlenmeyer flasks with screw caps.
- Gass funnels.
- Aid-washed glass wool.
- Balance capable of reading to four decimal places.
- Wrist Action Shaker.
- 10 mL, 50 mL, 100 mL, 1000 mL volumetric flasks.
- Pipettor or micro-pipettes for the preparation of working standards.
- Eppendorf pipettor (1-5 mL adjustable volume) or equivalent.
- 125 mL High Density Polyethylene (HDPE) storage bottles.
- Atomic Absorption Spectrophotometer.
- Graphite Tube Atomizer.
- Varian Partition Tubes (Coated) or equivalent.
- Hollow Cathode Lamps for: Ni, Pb, Cd, Cr, As, Se and Hg.
- Microwave Digestion System with temperature and pressure controls or equivalent.
- Advanced Composite Vessel (ACV) Digestion Vessel Assembly (X 2) or equivalent.
- Alternatively: Varian Axial Vista Simultaneous ICP or equivalent.
- Cetac U-5000AT+ Ultrasonic Nebulizer or equivalent.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade in quality.
- Concentrated HCl - trace metals analysis grade or equivalent.
- Concentrated HNO3 - trace metals analysis grade or equivalent.
- Type I water (as specified in ASTM D1193).
- Methanol.
- Hydrogen Peroxide (32 %).
- Ortho-phosphoric Acid - trace metals analysis grade or equivalent.
- Atomic Absorption Reference Standards - individual standards solutions at 1000 ug/mL.
Note: Reference standards must:- Come with a certificate of analysis.
- Be NIST traceable.
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
Important: The cleaning of glassware and the cleanliness of the environment in which the analysis is performed, directly effects the accuracy and precision of the method. In order to achieve accurate results, all glassware and digestion vessels must be cleaned immediately prior to use with dilute HCl (1+1) and then rinsed with Type I water.
8 Preparation of Solutions
- Nitric Acid Impinger Solution (10% HNO3 v/v )
- Add approximately 500 mL of Type I water to a 1000 mL volumetric flask.
- Add 100 mL of conc. HNO3.
- Make solution to volume with Type I water.
Note: When diluting a concentrated acid solution, it is always important to add the acid to water.
9 Preparation of Standards
- Elemental Standards and Required Dilutions
- All standards for graphite furnace analysis are made to a 10 % HNO3 (v/v) acid solution.
Note: For stability purposes, it is desired to dilute the analytical run standards in the same acid as the stock solution was purchased in. - All purchased stock standards are in 1000 ug/mL concentrations for stability purposes. The required standards for each analyte are found in the instrument parameters for each particular element in Appendix 1: Instrument Parameters.
- Representative dilutions are as follows:
- Primary Stock = 1000 µg/mL.
- Secondary Stock (As/Se) = 1mL of Primary Stock to 10 mL = 100 µg/mL.
- Mixed Stock :
= 100 µL of each Primary Stock (Pb, Ni, Cd) to 100 mL = 1 µg/mL each.
= 25 µL Cr Primary Stock to 100 mL = 0.25 µg/mL.
= 100 µL As/Se Secondary Stock to 100 mL = 0.10 µg/mL. - Standard 0 = 0 µL Mixed Stock to 100 mL.
- Standard 1 = 250 µL Mixed Stock to 100 mL.
- Standard 2 = 500 µL Mixed Stock to 100 mL.
- Standard 3 = 1500 µL Mixed Stock to 100 mL.
- Standard 4 = 3000 µL Mixed Stock to 100 mL.
- Standard 5 = 5000 µL Mixed Stock to 100 mL.
- All standards for graphite furnace analysis are made to a 10 % HNO3 (v/v) acid solution.
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars shall be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 with the following modifications as detailed below:
- Assemble the sidestream smoke train as per the diagram.
The following figure demonstrates sidestream apparatus using two impingers. The BAT fishtail chimney contains a mainstream glass filter pad which connects to the port of the smoking machine at its base; whereas the tygon tube is connecting the fishtail to the sidestream pad holder on the top of the chimney. The sidestream pad holder on the BAT fishtail chimney is connected by tygon tubing to two 70 ml impingers in series, each containing the extraction solution. The second impinger is then connected outwards by plastic tube connectors to a flow meter, and is also further connected to a pump that controls the flow rate of the exhaust.
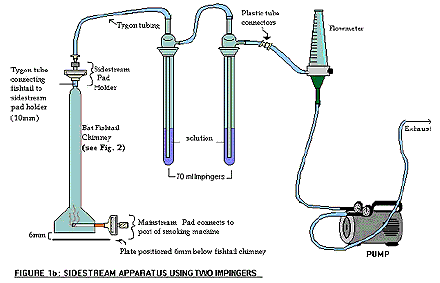
- The sidestream impingers are each loaded with 10 mL of a 10 % v/v HNO3 solution.
Note: A third empty impinger may be placed in series as an "overflow" to protect the flow meter. - Install the sidestream pad assembly at the top of the fishtail chamber and calibrate the vacuum pumps to draw at the rate of 3 L/minute. Record the flow meter settings.
- Assemble the sidestream smoke train as per the diagram.
- The machine conditions shall be as those specified in T-115 with the following modifications as detailed below:
13 Sample Generation
- Cigarettes shall be smoked and TPM collected as specified in T-115 with the following changes:
- Record the weights of the sidestream pad holders.
- Connect two clean 70 mL impingers in series each containing 10 mL of fresh 10 % HNO3.
- Place a third impinger (empty) to be used as a trap in case the impinger solution begins to overflow due to the "soapyness" of the resulting smoke into the impingers.
- Insert the first test cigarette to be smoked in position below the fishtail of the calibrated ports.
- Turn on the sidestream pumps and begin the lighting procedure at 30 seconds prior to lighting the cigarette.
- Light the cigarette on the first puff and then lower the fishtail assembly over the cigarette to a position of 6 mm above a plate that is beneath the cigarette. This is to create a uniform flow of air around the cigarette and up the fishtail chimney.
- Smoking is terminated and the butt is extinguished and removed when the cigarette has been consumed to the predetermined end mark.
- The pump continues for an additional 30 seconds to sweep any residual smoke up to the sidestream filter.
- The smoking process is repeated for the second, third and fourth cigarette.
- At the end of the smoking process, disassemble the sidestream apparatus and record the "after smoking" weights of the sidestream pad holders.
14 Sample Analysis
- Particulate Phase Sample Preparation and Digestion
- The first impinger's contents are transferred directly to a 125 mL Erlenmeyer flask. The particulate matter, trapped on the wall of the fishtail, is washed off using the second impinger solution. This rinse is added to the same Erlenmeyer flask.
- Rinse the second impinger with 10 mL of hydrogen peroxide, then transfer to the first impinger and subsequently the fishtail and Erlenmeyer.
- Repeat 14.1.2 using 10 mL of fresh impinger solution.
- The sidestream pad is added to the same Erlenmeyer flask.
- Extract the particulate matter from the pad on a wrist action shaker for 30 minutes.
- The mixture is transferred to a microwave digestion vessel by pouring it through a funnel with a plug of glass wool.
- The Erlenmeyer is then rinsed with an additional 2 X 10 mL of fresh impinger solution. These solutions are also added to the digestion vessel.
- Squeeze the residual solution trapped in the funnel using a glass rod so the liquid runs into the digestion vessel.
- Add 6 mL of concentrated HCl to the sample in the digestion vessel.
- Add 2 mL of concentrated HNO3 to the sample, swirling in the acid, and allow to sit until the original frothing subsides and there is no longer evidence of orange/brown fumes (NOx formation).
- Allow samples to sit until the effervescence subsides (approximately 10 minutes).
- Install the rupture membrane and cap the digestion vessel.
- Place the digestion vessel into the 12 places turntable and lock into position.
- Load the turntable of samples into the microwave digestor, and start the digestion program as described in Appendix 2: Microwave Program.
- When the digestion is complete, remove the turntable from the microwave and allow the samples to cool to room temperature before opening.
- Inspect the digestate. If the digestion appears to be incomplete, carefully add 2 to 4 more mL of hydrogen peroxide and return to the microwave for a secondary digestion as described in Appendix 2: Microwave Program.
- Transfer the digestate to a 100 mL volumetric flask and make to volume using the washings of the digestion vessel with Type I water.
- Transfer the contents of the flask into a 125 mL HDPE storage bottle.
Note: Samples should be stored in the highest concentration of both analyte and acid for stability purposes. Manual dilutions of the digestate should only take place at the time of analysis.
- Sample Dilutions required for Individual Elemental Analysis
- Samples may be required to be diluted so their absorbances fall within the desired calibration range with a good signal-to-noise ratio and very little matrix effect. Because of little matrix effect, standard additions is not required and a standards calibration will suffice.
- If samples must be diluted for analysis by graphite furnace atomic absorption, this dilution can be accomplished by adjusting the sample volume: blank ratio in the Sampler section of the method program.
Note: This dilution must be accounted for when calculating the results in a ng/cigarette basis. - The analysis of Cd and/or Pb may require a manual dilution prior to analysis by transferring 1000 uL of the digestate to a 10 mL volumetric flask, and making to volume with Type I water.
Note: When using ICP for quantitation, the samples may be analyzed without further dilution for Ni, Pb, Cd, and Cr.
Note: For As and Se, a multiple injection technique may be required for an adequate instrument response.
Note: These dilutions are based on "average" literature values that have been calculated in an indirect manner. These dilutions may need to be modified depending on: 1. the samples' country of origin, 2. the year in which the sample was grown (environmental factors), 3. the soil type and conditions which the sample was grown, 4. the type of tobacco used for the sample, 5. the stalk position of the tobacco used for analysis (if not a blended, finished product).
15 Atomic Absorption Analysis
- A, PB, CD, CR, AS, AND SE BY GRAPHITE FURNACE ATOMIC ABSORPTION
- Samples are analysed using the suggested parameters in Appendix 1: Instrument Parameters.
Note: Parameters may differ between instruments and must be optimized for the particular instrument used.
- Samples are analysed using the suggested parameters in Appendix 1: Instrument Parameters.
- Analysis of Ni, Pb, Cd and Cr by ICP-AES
- Samples are analysed using the suggested parameters in Appendix 3: ICP Parameters
Note: Parameters may differ between instruments and must be optimized for the particular instrument used.
- Samples are analysed using the suggested parameters in Appendix 3: ICP Parameters
- Calculations
Results reported by the instrument software are expressed as [ng/mL] in solution. This result, multiplied by the dilution of the sample and divided by the number of cigarettes smoked, will calculate the result in a [ng/cigarette] BASIS.- Analytical Result (on a "per cigarette" basis) :
Analyte [ng/cig] = (Analytical result [ng/mL] × 100 mL × Additional Dilution factor) / No. of Cigarettes.- The [ng/cigarette] results can be converted to [µg/g] by dividing this result by 1000.
- Total Particulate matter [mg/cigarette] is calculated using the difference in weight of the mainstream pad before and after smoking and dividing by the number of cigarettes smoked. This is used as a measure of reproducibility of the smoking procedure.
- Determination of Total Particulate Matter (TPM)
TPM [mg/cigarette]= [Wt. of MS Pad after smoking (g) - Wt. of MS Pad before smoking (g) ] × 1000 mg/g / 4.
Note: Particulate phase and gaseous phase metals are analyzed together.
- Analytical Result (on a "per cigarette" basis) :
16 Quality Control
- Each set analysis should contain one of each of the following per day of smoking or batch of up to 24 analyses (20-22 true samples):
- Laboratory Reagent Blank (LRB): to determine background contamination from solutions, glassware, or materials used in the analysis process.
- Laboratory Fortified Blank (LFB): to determine whether there is any loss of analyte as a result of the analysis process.
- Reference or Control Sample: to determine the inter-experimental reproducibility of the entire method of analysis
- Duplicate Sample: to determine the reproducibility of the procedure within the same experiment or batch on analysis.
Note: As an initial evaluation of the method and materials used, it is recommended that a minimum of 10 blanks be analysed using the method in order to establish control parameters for expected levels of background contamination before any samples are analysed. An LRB outside these control limits is an indicator of possible contamination problems or the use of materials and reagents of different lot numbers. It may be required to pre-wash the SS filter pads with a dilute acid solution in an attempt to leach out background metal contamination. In doing so, the filtering properties of the filter pad may change resulting in differences in SS TPM compared to other analysis.
- Recoveries and Levels of Contamination
- Recoveries for a Laboratory Fortified Blank (LFB) for Ni, Pb, Cd, and Cr are normally between 85 and 115 %. Variability in this range is associated to differences in the blanks.
- Recoveries for a Laboratory Fortified Blank (LFB) for As and Se range from 60 to 85 %. Lower recoveries result from over-heating of the sample while evaporating the methanol.
- Contamination must be monitored with each individual set of samples that are digested and is dependent on the laboratory environment. This ultimately effects the precision of the analysis.
- Method Detection Limit (MDL) / Limit of Quantitation (LOQ)
Note: Individual instruments will have different MDL's and LOQ's depending on the optimization of the instrument.- The MDL is defined as:
- The concentration of analyte that yields an absorbance of 0.004 units (the characteristic mass); or
- Determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The MDL is calculated as three times the standard deviation of these determinations; or
- As per item no. 2 analysing a blank a minimum of 10 times.
- The MDL (on a ng/cigarette basis) can be calculated by multiplying the determined MDL (ng/mL basis) by the final volume and dividing this by the number of cigarettes smoked.
- The MDL (on a ng/cigarette basis) can be enhanced by varying the amount of cigarettes smoked, however this may affect the amount of background contamination observed.
- The LOQ is defined as:
- The lowest standard used in the preparation of the calibration curve (excluding a blank); or
- Determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The LOQ is calculated as ten times the standard deviation of these determinations; or
- Same as per item no.two., using a blank solution.
- The LOQ (on a ng/cigarette basis) can be calculated by multiplying the determined LOQ (ng/mL basis) by the final volume and dividing this by the number of cigarettes smoked.
- The effect of modifying the number of cigarettes smoked and the volumes used for extraction and clean-up in the procedure on the LOQ is the same as for the MDL.
- The MDL is defined as:
- Stability of Reagents and Samples
- Secondary and Mixed Standards are stable for one week.
- Working standards must be prepared every other day.
- All samples must be analyzed within one week of the digestion or samples will have to be re-digested.
17 Modifications For Intensive Smoking
- No modifications are required for intense smoking conditions.
18 References
- Environmental Carcinogens - Selected Methods of Analysis, Vol. 8 - Some Metals: As, Be, Cd, Cr, Ni, Pb, Se, Zn. IARC Scientific Publication No. 71, 1986, p. 129-138.
- Perinelli, M.A. & Carugno, N., 1978. Determination of Trace Metals in Cigarette Smoke by Flameless Atomic Absorption Spectrometry, Beitrage zur Tabakforschung International, Band 9, Heft 4, Juli 1978, p. 214-217.
- Westcott, D.T. & Spincer, D., 1974. The Cadmium, Nickel and Lead Content of Tobacco and Cigarette Smoke, Beitrage zur Tabakforschung International, Band 7, Heft 4, April 1974, p. 217-221.
- Nitsch, Alfred et al, 1991. Schwermetalle in Tabaken und in Tabakrauch II: Spurenelemente Cadmium, Bleu, Kupfer, Kobalt, und Nickel in osterreischen Zigaretten und deren Rauchkondensaten und Rauchgasen, Beitrage zur Tabakforschung International, Volume 15, No. 1, August 1991, p. 19-32.
- Bell, Paul & Mulchi, Charles L., 1990. Heavy Metal Concentrations in Cigarette Blends, Tobacco Science, Vol. 34, 1990, p. 32-34.
- Varian Analytical Methods for Graphite Tube Atomizers, Varian Australia Pty Ltd, Publication No. 85-100848-00, 1988.
- Proctor, Christopher J. et al, Evaluation of an Apparatus Designed for the Collection of Sidestream Tobacco Smoke, Analyst Vol. 113, 1988, p. 1509-1513.
- Rhodes, Charles B and White, Ralph T., 1997. Mainstream Smoke Collection by Electrostatic Precipitation for Acid Dissolution in a Microwave Digestion System Prior to Trace Metal Determination, Journal of AOAC International, Vol. 80, No. 6, 1997, p. 1320-1331.
- Gawalco Et Al, 1997. Comparison of Closed-Vessel and Focused Open-Vessel Microwave Dissolution for Determination of Cadmium, Copper, Lead and Selenium in Wheat, Wheat Products, Corn Bran, and Rice Flour by Transverse- Heated Graphite Furnace Atomic Absorption Spectrometry, Journal of AOAC International, Vol. 80, No. 2, 1997, p. 379-387.
19 Appendices
Appendix 1: Typical Instrument Parameters
Graphite Furnace Atomic Absorption Analysis of: Ni
The following table lists the typical instrument parameters for graphite furnace atomic absorption analysis of specific chemicals. These parameters are grouped into method parameters as well as instrument parameters. The method parameters include the instrument, calibration and measurement mode. The instrument parameters include the lamp current in milliamps, slit width in nanometer, slit height, wavelength in nanometer, sample introduction, measurement, number of replicates and whether BGD correction is on. The parameters are listed for the following chemicals: nickel, lead, cadmium, chromium, arsenic and selenium.
Method Parameters:
Calibration Mode: Concentration
Measurement Mode: Peak Height
Instrument Parameters:
Slit Width (nm): 0.2
Slit Height: Normal
Wavelength: 232.0
Sample Introduction: Sampler Premixed
Measurement Time : 3.1
Replicates: 1
BGD Correction: On
Graphite Furnace Atomic Absorption Analysis of: Pb
Method Parameters:
Calibration Mode: Concentration
Measurement Mode: Peak Height
Instrument Parameters:
Slit Width (nm): 0.5
Slit Height: Normal
Wavelength: 283.3
Sample Introduction: Sampler Premixed
Measurement Time : 3.0
Replicates: 1
BGD Correction: On
Matrix Modifier: Ortho-phosphoric Acid (1000µg/mL)
Graphite Furnace Atomic Absorption Analysis of: Cd
Method Parameters:
Calibration Mode: Concentration
Measurement Mode: Peak Height
Instrument Parameters:
Slit Width (nm): 0.5
Slit Height: Normal
Wavelength: 228.8
Sample Introduction: Sampler Premixed
Measurement Time : 3.1
Replicates: 1
BGD Correction: On
Graphite Furnace Atomic Absorption Analysis of: Cr
Method Parameters:
Calibration Mode: Concentration
Measurement Mode: Peak Height
Instrument Parameters:
Slit Width (nm): 0.2
Slit Height: Reduced
Wavelength: 357.9
Sample Introduction: Sampler Premixed
Measurement Time : 3.2
Replicates: 1
BGD Correction: Off
Matrix Modifier: Ortho-phosphoric Acid (1000 µg/mL)
Graphite Furnace Atomic Absorption Analysis of: As
Method Parameters:
Calibration Mode: Concentration
Measurement Mode: Peak Height
Instrument Parameters:
Slit Width (nm): 0.2
Slit Height: Normal
Wavelength: 193.7
Sample Introduction: Sampler Premixed
Measurement Time : 3.0
Replicates: 1
BGD Correction: On
Matrix Modifier : Nickel Nitrate (100 µg/mL)
Graphite Furnace Atomic Absorption Analysis of: Se
Method Parameters:
Calibration Mode: Concentration
Measurement Mode: Peak Height
Instrument Parameters:
Slit Width (nm): 1
Slit Height: Normal
Wavelength: 196.0
Sample Introduction: Sampler Premixed
Measurement Time : 3.0
Replicates: 1
BGD Correction: On
Matrix Modifier : Nickel Nitrate (100 µg/mL)
Appendix 2: Microwave Digestion Parameters
Microwave Digestion Parameters
Model: MDS 2100
Digestion Vessel Type: ACV - Advanced Composite Vessels
The following table includes a summary of the microwave digestion parameters. These include the power, pressure, temperature, time of the program for the digestion of mainstream smoke samples in 5 stages.
| Stage: | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Power %: | 70 | 70 | 70 | 0 | 100 |
| Pressure (psi): | 50 | 125 | 175 | 20 | 150 |
| Run Time (min): | 20 | 10 | 30 | 20 | 20 |
| Time at Parameter: | 8 | 8 | 25 | 20 | 10 |
| Temperature: | 95 | 135 | 190 | 25 | 190 |
| Fan Speed | 50% | 50% | 50% | 80 |
Note: Both pressure and temperature are set as the controlling parameters in this digestion program. If the preset pressure or temperature is not reached, the microwave oven delivers the designated power for the time programmed in the Run Time function.
| Stage: | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Power %: | 75 | 75 | 75 | 0 |
| Pressure (psi): | 95 | 125 | 185 | 20 |
| Temperature: | 105 | 130 | 160 | 25 |
| Run Time (min): | 15 | 20 | 20 | 20 |
| Time at Parameter: | 10 | 15 | 15 | 20 |
| Fan Speed | 50 | 50 | 50 | 80 |
Note: These are only suggested parameters as a starting point. The digestion procedure must be optimized for the specific application and instrument used.
Appendix 3: ICP-AES Parameters
Plasma Flow (L/minute): 15.0
Auxiliary Flow (L/minute): 1.50
Nebulizer Flow (L/minute): 0.65
The following table provides the Inductively Coupled Argon Plasma – Atomic Emission Spectroscopy (ICP-AES) analysis parameters. Note that the emission wavelength differs between the four chemicals: nickel, lead, chromium and cadmium.
| Ni | Pb | Cd | Cr | |
|---|---|---|---|---|
| Emmision Wavelength (nm) | 221.648 | 220.353 | 214.439 | 267.716 |
Sample Introduction Settings
Pump rate (rpm): 20
Instrument Stabilization Delay(s): 15
Rinse Time(s): 10
General Settings
Replicate Read Time(s): 3.0
Number of Standards Defined: 5
Ultrasonic Nebulizer Set-up
Cooler: 2