Determination of Eugenol in Mainstream Tobacco Smoke
Health Canada
T-105 December 31, 1999
Table of Contents
- Scope of Application
- Normative references
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- Modifications for Intense Smoking Conditions
- Reference
- Appendices
1 Scope of Applications
- Applicable to the quantitation of Eugenol content in total particulate matter (TPM) of mainstream (MS) condensate.
- This method describes the separation and identification of eugenol in mainstream TPM from tobacco and clove cigarettes using reversed phase / isocratic High Performance Liquid Chromatography (HPLC) with ultra violet (UV) detection.
- The method is designed to be used as a routine analysis without the need for derivitization. This is applicable to processed cigarettes (Kreteks).
- This method is to only be used to determine the amount of eugenol added to tobacco as an flavourant.
- This method does not distinguish between the amount of eugenol added and the amount of naturally occurring eugenol (if any).
2 Normative References
- American Society for Testing Materials (ASTM) D 1193-77 - Standard Specifications for Reagent Water, Version 1977.
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Summary of Method
- Eugenol of mainstream tobacco smoke is collected by passing the smoke through a glass fiber filter disc (pad).
- The pad is then extracted with IPA standard solution.
- An aliquot is syringe filtered into a 1.5 mL amber autosampler vial and analyzed by HPLC with UV detection.
- Eugenol in MS TPM is quantified by external standard calibration procedures where the relative response of the samples are compared against a six point calibration.
- Time is a critical factor in eugenol analysis as conditioned clove cigarettes (kreteks) may lose a significant amount of clove oil in only a few days. After only one hour, an air- exposed glass fiber filter disc (pad) can lose 30 % of
eugenol added as a spike solution.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- Bottletop dispensor, 10-50 mL.
- Volumetric Flasks, amber - Class A - 10 mL, 100 mL, 10 L.
- Pipettes - Class A - 10 µL, 100 µL, 500 µL, 1 mL, 2.5 mL, 5 mL.
- Erlenmeyer flasks - 125 mL.
- Analytical Balance capable of measuring to 0.1 mg.
- Mechanical Shaker.
- Disposable Syringe - 5 cc.
- Glass Pasteur pipettes.
- Glass filtering funnel.
- Dessicant tube.
- Stirrer and stir bar.
- Parafilm® or equivalent.
- Syringe Filters - 0.45 µm PTFE (Teflon) 13 mm.
- PC controlled High Pressure Liquid Chromatography System consisting of:
- Solvent Delivery System - ternary gradient pump.
- Refrigerated Autosampler with partial fill sampling loop.
- UV Detector.
- Work Station.
- RP 18e Column.
- Disposable Guard Column.
6 Reagents and Supplies.
Note: All reagents shall be, at the least, recognized as analytical reagent grade in quality.
- Eugenol - purity > 99 %.
- Anethole - purity > 99 %.
- Methanol - Distilled in Glass (DIG).
- Isopropanol (IPA) - DIG.
- Water - Type I (meets ASTM D1193 specifications).
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner that contamination from glassware does not occur.
8 Preparation of Solutions
- Preparation of IPA Standard Solution
- Pipette 15 mL of methanol and 2000 µL of anethole (internal standard - ISTD) into a dry 10 L volumetric flask. Dilute to volume with IPA. This solution can also be used for water and nicotine determinations as in T-115. The volume can be adjusted to reflect quantities needed as long as the ratio of methanol and anethole to IPA remains the same.
- Store extraction solution in the dark at room temperature with a dessicant tube on the top and with slow, constant stirring.
9 Preparation of Standards
- Primary eugenol stock solution (2.0 mg/ml) is prepared by accurately weighing 200 mg of pure eugenol into a 100 mL volumetric flask and diluting to the mark with IPA standard solution.
- Six working standards in the range (2 to 1000 µg/mL) are prepared from the primary eugenol stock solution by dilution (0.01 to 5000µL) to 10 mL with IPA standard solution. (See Appendix 1).
- Transfer to 1.5 mL amber autosampler vials. Rinse vial first and then fill to minimize head space.
- Place vials in a vial file and store at 4 °C, protected from light until analyzed.
- Eugenol calibration standards are prepared fresh every five working days. Note: All solutions are stored at 4 °C.
10 Sampling
- The sampling of tobacco product for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars shall be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115.
13 Sample Generation
- Three cigarettes* shall be smoked and TPM collected as specified in T-115.
*For other tobacco products, select a number such that breakthrough does not occur.
14 Sample Analysis
- Extraction of filter pads
- For clove cigarettes: After smoking, slurry the pad in 50 mL of internal standard anethole stock solution (200 µg anethole/mL IPA) for 30 minutes in an Erlenmeyer flask on the mechanical shaker. Dilute 2 mL to 10 with the internal standard anethole stock solution for high yield cigarettes. Filter through the 0.45 µm filter into an autosampler vial.
- For non-clove cigarettes or light yield cigarettes: After smoking, slurry the pad in 40 mL of extraction solution (200 µg anethole/mL IPA) for 30 minutes in an Erlenmeyer flask on the mechanical shaker. Filter through the 0.45 µm filter into an autosampler vial.
- Reversed Phase HPLC Analysis
- UV Detector set to 280 nm.
- Chromatographic Conditions (Reversed Phase Analysis)
- Column Temperature: 30 °C.
- Mobile Phase: Reagents.
- Solvent A: Methanol : Type I water (80:20) filter and degas. (UHP Helium sparged).
- Sample Wash: Solvent A.
- Mobile Phase: Gradient
Flowrate: 0.7 mL/minute
Time (minute) Composition % A % B % C 0.0 100 0 0 20.0 100 0 0 Method End 100 0 0 (Equilibrate 10 minutes)
- Sample Analysis
- Sample vials are loaded onto the autosampler such that every 10th vial is a standard solution.
- Twenty µL of each sample vial is injected onto the HPLC column and analyzed. Elution pattern should be similar to Figure 1a, 1b.
- Calculations
- Construct a Calibration Curve:
- Twenty µL of each calibration standard is injected onto the HPLC and analyzed. Do in duplicate. Elution pattern should be similar to Figure 2.
- A calibration curve is prepared by plotting the concentration of eugenol vs. the peak area. Determine response factor from the calibration curve.
- Sample Quantification
- The amount of eugenol in whole tobacco samples is quantified by the external standard method.
- The identification of peaks is by comparison of retention times with standards, and the spiking of whole tobacco samples.
- Determination of Eugenol Deliveries in μg/cig
- Eugenol [μg/cig] ={[ Peak Area/ISTD] / Resp. Factor} × DF × {mL of Solution / # Cig. Smoked}
- Construct a Calibration Curve:
15 Quality Control
- Recoveries and Levels of Contamination
- Each analytical smoking run should also include:
- A Laboratory Reagent Blank (LRB) to evaluate the extent of any interference due to the glassware, trapping reagents, solvent and analyzer effects.
- A Laboratory Fortified Blank (LFB) to evaluate the extent of potential analyte loss.
- A Laboratory Fortified Matrix (LFM) to assess matrix interference. This is accomplished by spiking a true sample with a known concentration and determining a per cent recovery.
- Each analytical smoking run should also include:
- Method Detection Limit (MDL) and Limit of Quantitation
- Method Detection Limit (MDL)
- The method detection limit is determined by analyzing the lowest level standard at least 10 times as an unknown over several days. The MDL is then calculated as three times the standard deviation of these determinations.
- Limit of Quantitation (LOQ)
- The limit of quantification is determined by analyzing the lowest level standard at least 10 times as an unknown over several days. The LOQ is then calculated as 10 times the standard deviation of these determinations.
- Method Detection Limit (MDL)
- Stability of Reagents and Supplies
- All primary stock eugenol standards are prepared fresh weekly.
- All work standards, and extraction solvents are prepared fresh weekly.
- All samples are analyzed within 24 hours.
16 Modifications for Intense Smoking Conditions
- Under intense smoking conditions, the number of cigarettes smoked can be reduced to two.
17 Reference
- Myint, S., Daud, W.R.W., Mohmand, A.B., and Kadhum, A.A.H. Separation and Identification of Eugenol in Ethanol Extract of Cloves by Reversed-Phase High Performance Liquid Chromatography, Journal of American Oil Chemist Society, 72, 1995, p. 1231-1233.
- Guerin, M.R., Wise, M.B., and Holladay, S.K. Progress Report 3: Chemical Characteristics of Smoke Delivered by Clove-containing Cigarettes, NCI/ORNL/S&HP Topical Report #130, May 21 1985.
- Guerin, M.R., Wise, M.B., Holladay, S.K., and Jenkins, R.A. Progress Report 4: Deliveries of Clove Specific Constituents by U.S. Purchased Indonesian Clove-containing Cigarettes, NCI/ORNL/S&HP Topical Report #131, July 2 1985.
Appendices
The following table contains dilution information regarding 6 eugenol calibration standards. The table includes the volume of the primary eugenol standards, the final volume of the solution ( 10mL), and the final concentration of eugenol in each standard.
| Standard ID | Vol (ml) 1o Eugenol | Final Volume in mL | Eugenol [µg/mL] |
|---|---|---|---|
| Std 1 | 3.0 | 10 | 562.8 |
| Std 2 | 1.5 | 10 | 281.4 |
| Std 3 | 0.7 | 10 | 131.2 |
| Std 4 | 0.300 | 10 | 56.28 |
| Std 5 | 0.150 | 10 | 28.14 |
| Std 6 | 0.030 | 10 | 5.628 |
Note 1: The concentration of Eugenol will vary depending on the exact concentration of primary stock prepared.
Figure 1a. Overlay Chromatogram of MS TPM extract sample (Kretek type cigarette) and calibration standard. (Eugenol peak is at RT 4.644 minutes, Anethole Internal Standard is at 10.725 minutes).
This figure is an overlay chromatogram of MS TPM extract sample (Kretek type cigarette) and calibration standard. The Eugenol peak is at RT 4.644 minutes, Anethole Internal Standard is at 10.725 minutes.
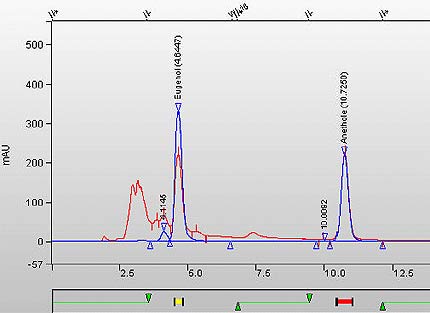
Figure 1b. Overlay Chromatogram of whole tobacco (1R4F cigarette type) and calibration standard. (Note the absence of eugenol peak in the sample at RT 4.672 minutes).
This figure is an overlay chromatogram of whole tobacco (1R4F cigarette type) and calibration standard. Note that the eugenol peak in the reference cigarette sample is absent at RT 4.672 minutes.
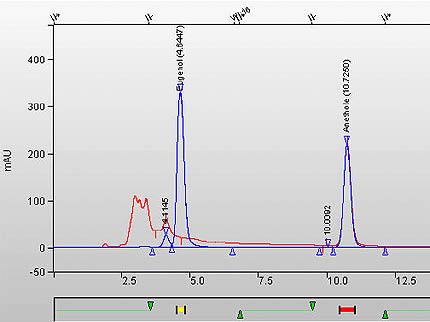
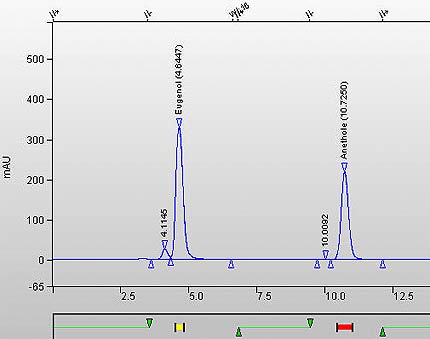
Figure 2. Chromatogram of Eugenol Calibration Standard. (Eugenol RT is 4.6447 minutes and Anethole ISTD is at 10.726 minutes).
The following figure is a chromatogram of Eugenol Calibration Standard. The retention time for Eugenol is 4.6447 minutes, and for Anethole ISTD is at 10.726 minutes.