Determination of Nitrosamines in Mainstream Tobacco Smoke
Health Canada
T-111 December 31, 1999
Table of Contents
- Scope of Applications
- Normative references
- Definitions
- Method Summary
- Apparatus
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- Modifications for Intense Smoking Conditions
- Reference
- Appendices
1 Scope of Applications
- Applicable to the determination of tobacco specific nitrosamines (TSNA) in mainstream tobacco smoke.
- The volatile nitrosamines (VNA) are formed during the processing of tobacco and during the smoking of tobacco products. The tobacco processing methods include air-, sun-, flue- and fire-curing, aging and fermentation.
- The generation of mainstream smoke is achieved under standard machine smoking conditions for cigarettes as specified in T-115.
- This method is suitable for the quantitative determination of four tobacco specific N-nitrosamines in mainstream (MS) tobacco smoke only: N-nitrosonornicotine (NNN), 4-(N-nitrosomethylamino)- I - (3-pyridyl)- 1 -butanone (NNK), N-nitrosoanatabine (NAT) and N-nitrosoanabasine (NAB).
2 Normative References
- Health Canada Test Method T-115 Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- Mainstream smoke of 10 cigarettes* is trapped onto a 92 mm glass fibre filter disc (pad). The TSNA are concentrated by extraction with dichloromethane, followed by column chromatography onto basic Alumina. The fraction containing TSNA is eluted, then quantitatively analysed by combined gas chromatography-thermal energy analysis (GC-TEA). N-nitrosoguvacoline (NG) is used as an internal standard.
*For other tobacco products, select a number such that breakthrough does not occur.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
All four TSNA are carcinogenic in several species of laboratory animals. Extreme care should be taken in handling these compounds. The exhaust of the TEA detector should be vented properly in order to reduce exposure to possible excess ozone (03).
5 Apparatus
- Equipment needed to perform smoking of tobacco product as specified in T-115.
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- 250 mL Round-bottom flask with ground glass joint.
- Volumetric Flasks - 2,10, 50,100 mL.
- Amber flask or storage bottle.
- Aluminum foil.
- Silanized glass wool.
- Burrell wrist action shaker or equivalent.
- LC Column with Frit and Stopcock, 300 mm × 22 mm ID × 25 mm OD (Supelco 64754) or equivalent.
- Short Stem Glass Funnels.
- Glass Pasteur Pipettes.
- Zymark TurboVap II Concentrator equipped with 200 mL Tubes with Graduated 1 mL stem or equivalent.
- Thermal Energy Analyzer (Thermo-Electron Corp.) interfaced to GC or equivalent.
- Gas chromatograph (GC), equipped with temperature programmable injector and electronic flow control.
- GC column - 30 m × 0.32 mm × 3.0 µm DB-1 fused silica capillary column.
- Non-ultra violet (UV) lighting.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade in quality.
- Dichloromethane (DCM) -Distilled in Glass (DIG).
- Acetone - DIG.
- Methanol - DIG.
- Basic alumina.
- Aluminum foil.
- Glass fibre filter pads - 92 mm with holders.
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
8 Preparation of Solutions
- 1:1 Acetone:Dichloromethane solution
- Mix Acetone and Dichloromethane together in a 1:1 v/v basis.
9 Preparation of Standards
- N-nitrosoguvacoline (NG) internal standard (ISTD)
- Prepare a solution at 5000 ng/mL in dichloromethane.
- TSNA mixed standard solution
- Prepare a mixed standard dilution stock solution of NNN, NAT, NAB and NNK in dichloromethane at the following range of concentrations:
- NNK at 3000 ng/mL.
- NNN and NAT at 1500 ng/mL.
- NAB at 500 ng/mL.
- Store this solution in an amber flask and protect from light.
Note: Concentrated solutions are stable for at least six months if stored at - 20 °C in such a manner as to prevent loss of solvent from evaporation. - Build a calibration curve ranging from 20 ng/mL (for NAB) to 2000 ng/mL (for NNK) containing NG as an internal standard at 500 ng/mL in each of the standards.
Note: Individual calibration stocks are stable for two months if stored at - 20 °C in such a manner as to prevent loss of solvent from evaporation.
- Prepare a mixed standard dilution stock solution of NNN, NAT, NAB and NNK in dichloromethane at the following range of concentrations:
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product is to be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars are to be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Non-UV lighting shall be used in the rooms in which sample generation and sample analyses are conducted.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 for a rotary machine.
13 Sample Generation
- Samples shall be smoked and TPM collected as specified in T-115 with the following modifications:
- Place 10 cigarettes (or little cigars), one in every other position, in the smoking machine and smoke the samples.
- After smoking 10 cigarettes (or little cigars), perform three clearing puffs and remove the pad holder from smoking machine.
14 Sample Analysis
- Extraction of Filter Pads
- Remove the pads from the holders and place each into a 250 mL round bottom flask wrapped with Aluminum foil.
- Add 200 µL of NG internal standard solution onto each pad (an amount equivalent to the concentration of NG in the standards on a ng/mL basis).
- Add 100 mL dichloromethane to each flask.
- Extract on a wrist action shaker for 30 minutes.
- Sample Concentration
- Filter out the pad material from the extract using a plug of silanized glass wool placed in a short stem funnel directly into a 200 mL Zymark tube.
- Rinse the flask with 20 mL DCM. After the initial extract has dripped through the funnel, add the rinse to the funnel.
- Repeat Step 14.2.2.
- After all the solution has stopped dripping through the funnel, place the sample into the TurboVap II Concentrator set at 38°C and 10psi nitrogen.
- Concentrate samples to approximately 5 mL.
- Column Chromatography Clean-up Procedure
- Prepare a basic alumina column by adding 50 mL of DCM to an empty, dry, glass LC column.
- Add 10 g (+/- 0.2 g) of oven dried (110°C) basic alumina to the liquid in the column. Stir the alumina slurry with a glass rod to remove any possible air pockets.
- Drain the liquid from the column to waste, closing the stopcock when the solution is at the level of the alumina.
- Wash the alumina by adding 50 mL of DCM to the column. Discard the liquid and close the stopcock when the solution is at the level of the alumina.
- Add the 5 mL sample from the TurboVap tube to the alumina column using a glass Pasteur pipette attempting not to disturb the alumina packing.
- Discard the liquid from the column, close the stopcock when the solution is at the level of the alumina.
- Rinse the TurboVap tube with 10 mL DCM, washing the lower portion (25 %) of the tube with repeated flushing using a Pasteur pipette.
- Add the 10 mL rinse from the TurboVap tube to the alumina column using a glass Pasteur pipette attempting not to disturb the alumina packing.
- Discard the liquid from the column, close the stopcock when the solution is at the level of the alumina.
- Rinse the TurboVap tube and alumina column with an additional 40 mL DCM attempting not to disturb the alumina packing, draining the liquid from the column to waste.
- Place a clean 200 mL TurboVap tube beneath the LC Column to collect the sample (eluate).
- Elute the TSNA from the Alumina column by adding 50 mL of 1:1 Acetone:DCM to the column attempting not to disturb the Alumina packing.
- Collect the liquid from the column into the TurboVap tube, closing the stopcock when the solution is at the level of the Alumina.
- Repeat steps 14.3.12 and 14.3.13, four more times, collecting the eluate in the same TurboVap tube (a total of 250 mL collected).
Note: This will require evaporating a portion of the eluate on the TurboVap before the final 50 mL can be collected into the tube.
- Sample Re-Concentration
- Place the samples into the TurboVap II Concentrator set at 38 °C and 9 psi nitrogen.
- After the samples have been concentrated to approximately 150 mL, increase the pressure to 10 psi.
- Concentrate samples to 0.8 mL or until the sensor turns the concentration off (approximately 45 minutes).
- Transfer the concentrate and rinse to a 2 mL volumetric flask and make to volume with 1:1 Acetone:DCM, using the solvent to perform a rinse of the TurboVap tube.
- Transfer the contents of the flask to an amber autosampler vial with Teflonlined septa for GC analysis.
- GC-TEA Operating Conditions
- Carrier flow rate (He): 2.8 mL/minute using electronic flow control (velocity = 60 cm/second).
- Injector temperature: programmable 35 to 220 °C.
- Oven temperature: programmed 50 to 170 to 212 °C.
- TEA interface temperature: 240 °C.
- TEA furnace temperature: 500-525 °C (dependent on analyzer sensitivity).
- Analysis Run Time: 35 minutes.
- Blank test
- Blank tests using purified nitrosamine-free air should be performed periodically in order to ensure the absence of nitrosamine traces in the analytical environment, or their formation during analysis.
- GC-TEA calibration
- Inject 1.5 µL of the TSNA mixed standard solution and determine peak areas for the four components.
- TSNA determination
- Inject 1.5 µL of the sample concentrate (12.4.5) and determine areas of the peaks having retention times corresponding to NNN, NAT, NAB and NNK.
- Note: See Appendix 1 for representative chromatograms.
- Calculations
- The content, mcig (ng/cigarette), of a given TSNA is obtained from:
mcig = CVs/N.
where
C = analytical Concentration determined by ISTD calibration of given TSNA.
Vs = final volume of concentrate.
N = number of cigarettes/cigarette equivalents/little cigars/cigars/ kreteks/bidis smoked.
- The content, mcig (ng/cigarette), of a given TSNA is obtained from:
15 Quality Control
- Typical Chromatograms
- See Appendix 1.
- Method Detection Limits (MDL) / Limit Of Quantitation (LOQ)
- The method detection limit (MDL) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The MDL is calculated as three times the standard deviation of these determinations.
- The MDL (on a ng/cigarette basis) can be calculated by multiplying the determined MDL (ng/mL basis) by the final volume of the extract and dividing this by the number of cigarettes smoked.
- The MDL (on a ng/cigarette basis) can be enhanced by modifying the number of cigarettes smoked and the volumes used for extraction and clean-up in the procedure.
- The practical limit of quantitation (LOQ) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The LOQ is calculated as 10 times the standard deviation of these determinations.
- The LOQ (on a ng/cigarette basis) can be calculated by multiplying the determined LOQ (ng/mL basis) by the final volume of the extract and dividing this by the number of cigarettes smoked.
- The effect of modifying the number of cigarettes smoked and the volumes used for extraction and clean-up in the procedure on the LOQ is the same as for the MDL.
16 Modifications for Intense Smoking Conditions
- Under intense smoking conditions, the number of cigarettes and/or the final solution volumes may be modified to maintain the same calibration range as with standard smoking conditions.
17 Reference
- Fisher, S., and Spiegelhalder, B. Improved Method for the Determination of Tobacco Specific Nitrosamines (TSNA) in Tobacco Smoke, Beitrage zur Tabakforschung International, 14, 1989, p. 145-153.
- Fisher, S., Castonguay, A., Kaiserman, M., Spiegelhaider, B., and Preussmann, R. Tobacco-specific nitrosamines in Canadian cigarettes, J. Cancer Res. Clin Oncol., 116, 1990, p. 563-568.
- Adams, J.D., Brunnemann, K.D. & Hoffmann, D. Chemical studies on tobacco smoke. LXXV. Rapid method for the analysis of tobacco-specific N-nitrosamines by gas-liquid chromatography with a thermal energy analyser, J. Chromatogr., 256, 1983, p. 347-351.
Appendices
Appendix 1: Typical Chromatograms
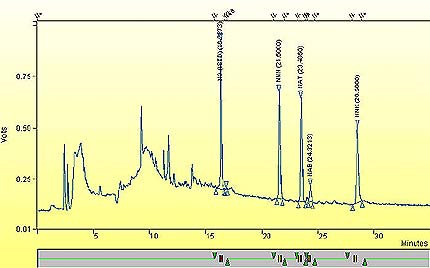
A representative chromatogram of Std 4.
This figure demonstrates a representative sample of a chromatogram of Standard 4 of TSNAs.
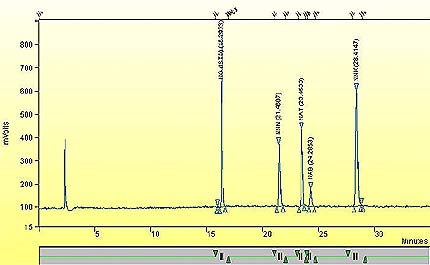
A representative chromatogram of 1R4F mainstream smoke.
This figure demonstrates a sample representative chromatogram of 1R4F mainstream smoke.