Determination of 1- and 2- Aminonaphthalene and 3- and 4- Aminobiphenyl in Sidestream Tobacco Smoke
Health Canada
T-202, December 31, 1999
Table of Contents
- Scope of Applications
- Normative References
- Definitions
- Summary of Method
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- References
- Appendices
1 Scope of Applications
- Applicable to the isolation and quantitation of the aromatic amine content (1- and 2-aminonaphthalene and 3- and 4-aminobiphenyl) of sidestream tobacco smoke.
2 Normative References
- American Society for Testing and Materials (ASTM) D1193-77 - Standard Specifications for Reagent Water, Version 1977.
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Summary of Method
- This method is used for the analysis of sidestream (SS) tobacco smoke using a fishtail chimney configuration. Sidestream smoke is all the smoke emitted from the lit end of a burning cigarette during the smoulder process. The glass fishtail chimney sits over a burning cigarette and allows the smoke to be directed in a controlled manner for the determination of sidestream tobacco constituents.
- Aromatic amines of sidestream tobacco smoke are collected by passing the smoke from two cigarettes through a glass fibre filter disc (pad). The pad is placed in an Erlenmeyer flask with 100 mL of 5 % hydrochloric acid solution. The flask is shaken for 30 minutes on a wrist-action shaker and the contents filtered into a 500 mL separatory funnel. The internal standard (2H9-4-aminobiphenyl) is spiked into the solution. The extract is washed with dichloromethane, made basic with sodium hydroxide solution and extracted with hexane. The hexane extracts are dried with sodium sulfate, derivatized with pentafluoropropionic acid anhydride and trimethylamine, passed through a florisil column, and quantitated by GC/MS.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- Volumetric Flasks - Class A - 10 mL, 25 mL, 100 mL.
- Pipettes - Class A - 20 μL, 50 μL, 100 μL, 250 μL, 500 μL, 1 mL or equivalent gas-tight syringe covering the range required.
- Graduated Cylinder - 50 mL, 100 mL.
- Fish-tail Chamber (mounted on a retort stand).
- 125 mL Polymethylpentene (PMP) Erlenmeyer flasks with screw-cap (or equivalent).
- Separatory Funnels - 500 mL with glass stoppers.
- Filter funnels - 10 cm internal diameter.
- Round-bottom flasks - 500 mL.
- Pasteur pipettes (disposable) with rubber bulbs.
- Conical tubes - 15 mL.
- Autosampler vials and caps with Teflon-lined septa (disposable).
- Analytical balance measuring to at least four decimal places.
- Vacuum pump (GAST or equivalent).
- Victor flow meter calibrated at three litres per minute.
- Tygon tubing.
- Glass fibre filter pad holders and glass fibre filter pads.
- Rotary Evaporator - with water bath set at 40 °C.
- Turbo Evaporator - with water bath set at 40 °C.
- Supelco SPE-system or equivalent.
- GC/MS System - Autosampler, SPI Injector with high-performance insert, GC, Ion Trap Detector or equivalent.
- GC Column - FS-capillary DB-5MS, 30 m X 0.25 mm ID X 0.25 μm or equivalent.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade quality.
- D9-4-aminobiphenyl.
- 1-Aminonaphthalene - 95 % purity or better.
- 2-Aminonaphthalene - 95 % purity or better.
- 4-Aminobiphenyl - 98 % purity or better.
Note: 3-aminobiphenyl is not available.
- Hydrochloric Acid - 32 %.
- Hexane - Distilled in Glass (DIG).
- Dichloromethane - (DIG).
- Diethylether - (DIG).
- Benzene - (DIG).
- Acetone - (DIG).
- Water - Type I (meets ASTM D 1193 specifications).
- Sodium Hydroxide Solution - 50 %.
- Sodium Sulfate - Granular.
- Florisil SPE Columns (Supelco) - 6 mL X 1 g packing or equivalent.
- Florisil (J.T.Baker) - deactivated 60-100 mesh or equivalent.
- Pentafluoropropionic Acid Anhydride (PFPA).
- Trimethylamine - 40 % wt solution in water.
- pH Paper - high range.
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
8 Preparation of Solutions
- 5 % Hydrochloric Acid - Add 312 mL of 32 % HCl solution to 1 L of Type I water, dilute to 2 L with Type I water. Mix very well.
- TMA Solution - Add 2 mL of 40 % Trimethylamine Solution to a conical tube containing 2 mL of hexane. Vortex for one minute, let settle and transfer hexane to a 1.5 mL autosampler vial. Cap and refrigerate when not in use.
- Florisil Elution Solution - 500 mL hexane, 400 mL benzene and 100 mL acetone. Mix well.
9 Preparation of Standards
- Stock Solutions
- A primary stock solution (1 mg/mL) is prepared by accurately weighing 25 mg of pure 4-aminobiphenyl (4-amb) into a 25 mL volumetric flask and diluting to volume with diethylether.
- A primary stock solution (1 mg/mL) is prepared by accurately weighing 25 mg of pure 1-aminonaphthalene (1-amn) into a 25 mL volumetric flask and diluting to volume with diethylether.
- A primary stock solution (1 mg/mL) is prepared by accurately weighing 25 mg of pure 2-aminonaphthalene (2-amn) into a 25 mL volumetric flask and diluting to volume with diethylether.
- A mixed secondary stock solution is prepared by diluting the 100 μL of primary 4-amb solution, 500 μL of the primary 1-amn solution and 500 μL of primary 2-amn solution to 10 mL with hexane.
- A tertiary stock solution is prepared by diluting 500 μL of the secondary stock solution to 25 mL with hexane. This solution is approximately 200 ng/mL in 4-amb and 1000 ng/mL in the aminonaphthalenes.
- Internal Standard Solution (D9-4-aminobiphenyl)
- A primary stock solution (100 μg/mL) is prepared by accurately weighing 10 mg of pure D9-4-aminobiphenyl (D9-4amb) into a 100 mL volumetric flask and diluting to volume with hexane.
- Internal Standard Spiking Solution (200ng/mL D9-4amb) (ISTD)
- The internal standard spiking solution is prepared by diluting 100 μL of the primary D9-4-aminobiphenyl stock solution to 50 mL with hexane.
- Working Standards
- Standard 1 (80 ng/mL 4-amb & 400 ng/mL 1- and 2-amn) - Add 4 mL of tertiary stock solution and 1mL of internal standard spiking solution to a 10 mL volumetric flask and make to the mark with hexane. Mix well. Transfer 1 mL of this solution to a conical tube, add 50 μL of TMA, 50 μL of PFPA, vortex and let sit a minimum of 30 minutes. Proceed as under sample preparation from 14.2.2.
- Standard 2 (40 ng/mL 4-amb & 200 ng/mL 1- and 2-amn) - Add 2 mL of tertiary stock solution and 1mL of internal standard spiking solution to a 10 mL volumetric flask and make to the mark with hexane. Mix well. Transfer 1 mL of this solution to a conical tube, add 50 μL of TMA, 50 μL of PFPA, vortex and let sit a minimum of 30 minutes. Proceed as under sample preparation from 14.2.2.
- Standard 3 (20 ng/mL 4-amb & 100 ng/mL 1- and 2-amn) - Add 1 mL of tertiary stock solution and 1 mL of internal standard spiking solution to a 10 mL volumetric flask and make to the mark with hexane. Mix well. Transfer 1 mL of this solution to a conical tube, add 50 μL of TMA, 50 μL of PFPA, vortex and let sit a minimum of 30 minutes. Proceed as under sample preparation from 14.2.2.
- Standard 4 (10 ng/mL 4-amb & 50 ng/mL 1- and 2-amn) - Add 0.5 mL of tertiary stock solution and 1 mL of internal standard spiking solution to a 10 mL volumetric flask and make to the mark with hexane. Mix well. Transfer 1 mL of this solution to a conical tube, add 50 μL of TMA, 50 μL of PFPA, vortex and let sit a minimum of 30 minutes. Proceed as under sample preparation from 14.2.2.
- Standard 5 (5 ng/mL 4-amb & 25 ng/mL 1- and 2-amn) - Add 0.25 mL of tertiary stock solution and 1 mL of internal standard spiking solution to a 10 mL volumetric flask and make to the mark with hexane. Mix well. Transfer 1 mL of this solution to a conical tube, add 50 μL of TMA, 50 μL of PFPA, vortex and let sit a minimum of 30 minutes. Proceed as under sample preparation from 14.2.2.
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars shall be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 with the following modifications as detailed below:)
- Smoking is conducted on four to eight ports of a linear 20 port smoking machine.
The following figure demonstrates sidestream apparatus. The BAT fishtail chimney contains a mainstream pad which connects to the port of the smoking machine at its base; whereas the tygon tube is connecting the fishtail to sidestream pad holder on the top of the chimney. The sidestream pad holder on the BAT fishtail chimney is connected to the flow meter which is further connected to a pump that controls the flow rate of the exhaust.
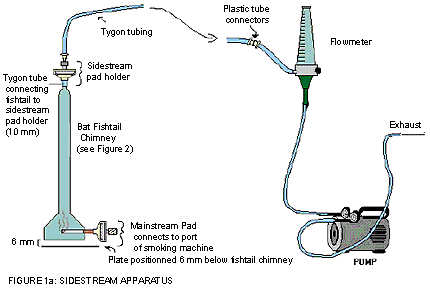
- Smoking is conducted on four to eight ports of a linear 20 port smoking machine.
- The machine conditions shall be as those specified in T-115 with the following modifications as detailed below:)
13 Sample Generation
- The mainstream smoke of two cigarettes* is passed through a pad attached to the port and the Total Particulate Matter (TPM) is determined.
- The sidestream smoke is drawn up from the cigarettes, through the fishtail chamber and a cassette holding a pre-weighed glass fibre filter pad.
- After smoking, weigh the SS filter holder for TPM, and place pad in an Erlenmeyer flask. Rinse the fishtail chimney into the flask with 100 mL of 5 % HCl, and cap. Store, until extracted, in a refrigerator at 4 °C.
*For other tobacco products, select a number such that breakthrough does not occur.
14 Sample Analysis
- Liquid-Liquid Extraction
- Mix sample well on a wrist-action shaker for 30 minutes.
- Filter the contents of the flask through a filter funnel with a glass wool plug into a separatory funnel. Rinse the flask three times with 5 % HCl and transfer rinsing into the separatory funnel.
- Add 100 μL of the ISTD solution to the separatory funnel. Stopper and shake.
- Add 50 mL of dichloromethane to the separatory funnel. Shake with careful venting until there is no more pressure and let contents settle.
- Draw off the dichloromethane layer into a beaker and discard as chlorinated waste.
- Repeat 14.1.4 and 14.1.5 twice (total of three rinses).
- Slowly add 10 to 15 mL of 50 % NaOH solution to the aqueous phase in the separatory funnel. Mix gently with careful venting, until venting no longer releases pressure, and check that the pH is above 12. If it is not, add 5 mL more NaOH. If more than 20 mL of NaOH need to be added, check that the HCl solution was made up properly.
- Add 50 mL of hexane to the separatory funnel and shake VERY, VERY carefully with venting into the fume-hood. Shake until there is no more pressure and let contents settle.
- Prepare a filter funnel with a plug of glass wool and approximately 100 g of sodium sulphate. Rinse the sodium sulphate with approximately 50 mL of hexane into a 500 mL round-bottom flask. Discard the rinse.
- Draw bottom (aqueous) layer of the contents of the separatory funnel into a beaker and retain.
- Pass the top (hexane) layer through the sodium sulphate into the 500 mL round bottom, spreading the hexane over the entire surface of the sodium sulphate.
- Pour the aqueous layer in the beaker back into the separatory funnel and add 50 mL of hexane. Shake very carefully and let settle.
- Repeat 14.1.10 to 14.1.12.
- After the third extraction with hexane, the aqueous layer can be discarded.
- Repeat 14.1.11.
- Rinse the sodium sulphate with approximately 50 mL hexane into the round-bottom flask.
- Add 50 μL of the TMA solution and 50 μL of the PFPA. Swirl and observe that a fine mist forms in the flask. Let sit for a minimum of 30 minutes (or overnight).
- Solid Phase Extraction (SPE)
- Evaporate the sample in the round bottom flask on the rotary evaporator to near dryness.
- Prepare a florisil column by adding 2 g of florisil to a 6 mL X1 g florisil tube. Add a small amount of sodium sulfate to the top of the florisil.
- Pre-wash with 5 mL of hexane: benzene: acetone (removing all air bubbles from the column).
- Add approximately 1 mL of the hexane: benzene: acetone solution to the round-bottom flask (rinsing the solution down the sides of the flask).
- Transfer the solution to the top of the florisil column and let drain to the top of the florisil into a 15 mL conical tube.
- Repeat 14.2.4 and 14.2.5 two more times.
- Rinse the florisil tube with hexane: benzene: acetone until approximately 15 mL have been collected in the conical tube.
- Reduce the volume of the eluate in the tube in the TurboVap Evaporator under a gentle stream of nitrogen (3 to 5psi) until approximately 1 mL remains.
- Make the volume in the tube up to approximately 1 mL if necessary with hexane and vortex briefly (approximately 10 seconds).
- Transfer the contents to an autosampler vial, cap and store at 4°C until injected on the GC/MS.
- GC/MS Operating Conditions
- Injector Temperature: 60 °C for 0.5 minute.
180 °C per minute to 280 °C, hold to end of run. - Column Temperature: 80 °C for two minutes.
10 °C per minute to 210 °C.
20 °C per minute to 280 °C, hold at 280 °C for 3.5 minutes. - Transfer Line Temperature: 270 °C.
- Manifold Temperature: 240 °C.
- Column Head Pressure: 12 psi.
- Injection Volume: 1 μL.
- Scan Range: 100 to 330 amu .
- Ion Peaks Used: m/z 315 for analyte (3- and 4- aminobiphenyl).
m/z 324 for internal standard (D9-4 aminobiphenyl).
m/z 289 for analyte (1- and 2- aminonaphthalene).
- Injector Temperature: 60 °C for 0.5 minute.
- A calibration curve (ratio of each analyte's response to the ISTD response versus the amount of the analyte in ng/mL) is generated at the beginning of analysis from the five working standards. Quantitation is performed using the internal standard method available with the GC/MS software. A calibration curve for 3-aminobiphenyl is generated from the calibration for 4-aminobiphenyl. The spectra and retention times for these two analytes are established with the analysis of a control cigarette.
- A Check Standard is run every 20 injections and is analyzed as a sample to confirm that the calibration is still valid. If the result differs by more than 10 % of the expected value for that standard, the calibration process must be repeated and a new calibration curve generated.
- The amount of each analyte is reported in ng/cigarette and is calculated as follows:
Analyte (ng/cigarette) = [Amount of Analyte from Curve (ng/mL) * Final Volume (1mL)] / Number of Cigarettes
Note: There is no volume dilution factor because all of the samples are concentrated to the final stage. The samples and standards are quantitated in the same manner.
15 Quality Control
- Typical Chromatograms
- See Appendix.
- Recoveries and Levels of Contamination
- With every set of approximately 20 samples, include a laboratory reagent blank (LRB). The LRB is the pad plus extraction solution (100 mL of 5 % HCl) spiked with ISTD solution and taken through the sample preparation to determine if any of the reagents contribute to the aromatic amines. LRB results are consistently ND (not detected) for all analytes.
- With every set of approximately 20 samples, include a laboratory fortified blank (LFB). A known amount of the tertiary stock solution is added to solution in a separatory funnel along with the ISTD and the solution is taken through the entire procedure, to determine if any aromatic amine is lost during the different stages. Recoveries of 2- aminonaphthalene and 4-aminobiphenyl are typically 90 ± 10 %. Recoveries of 1-aminonaphthalene are somewhat lower (70 to 80 %) due to the more volatile nature of the derivatized 1-aminonaphthalene.
- To assess potential matrix interference, a laboratory fortified matrix (LFM) can be analyzed. A sample of the control cigarette can be split before extraction and each half treated as a separate sample. One of the split halves should be spiked with a known amount of the tertiary stock solution at approximately the level expected in the sample. Recoveries should be very close to 100 %. Typical recoveries are:
4-aminobiphenyl - 98.4 %.
2-aminonaphthalene - 96.8 %.
- Method Detection Limit (MDL) and Limit of Quantitation(LOQ)
- For GC/MS analysis, the detection limit can be defined as a peak whose signal to noise ratio (S/N) is three to one. The limit of quantitation can be defined as a S/N of 10 to one. The lowest standard typically run for 4- aminobiphenyl is 1 ng. This peak gives a S/N of approximately 20 to one and a corresponding MDL of approximately 0.08ng/cigarette and an LOQ of approximately 0.25 ng/cigarette for a smoking run of two cigarettes.
- Stability of Reagents and Samples
- Store ISTD spiking solution in a 25 mL amber vial with open cap and Teflon-lined septum. Use a 100 μL gas-tight syringe to transfer the ISTD from the vial to the separatory funnel. Do not touch the sides of the separatory funnel with the tip of the syringe (to avoid contaminating the ISTD). Wash the syringe with hexane between samples and change the septum daily. Store the ISTD at 4 °C when not in use.
- There is no apparent problem with the stability of the stock or underivatized working standards. Derivatize fresh 1 mL aliquots of the working standards weekly and replace the red Teflon-lined septa after each injection to minimize contamination from the septa.
- Samples should be extracted within one week of being produced.
- The area responses of the analytes (including the internal standards) in the calibration standards and the LFBs are occasionally lower than in the samples and LFMs. Investigation has suggested that analyte loss occurs if the standards or LFBs are taken to complete dryness during rotary evaporation or turbo evaporation. The samples do not appear to be affected in the same way, possibly due to the matrix acting as a "keeper" during the solvent removal stages. As a cautionary measure, no samples or standards are taken to complete dryness at any stage of the process.
16 References
- Pieraccini, G., F. Luceri, and G. Moneti, 1992. New Gas-Chromatographic/Mass- Spectrometric Method for the Quantitative Analysis of Primary Amines in Main and Sidestream Cigarette Smoke. I. Rapid Communications in Mass Spectrometry. 6, p. 406-409.
17 Appendices
Figure 1: Total Ion Chromatogram (TIC) for a Control Cigarette and ion chromatograms for the aminonaphthalenes (m/z 289), aminobiphenyls (m/z 315) and D9-4-aminobiphenyl (m/z 324).
The following image displays a total ion chromatogram (TIC) for a control cigarette and ion chromatograms for the aminonaphthalenes (m/z 289), aminobiphenyls (m/z 315) and D9-4-aminobiphenyl (m/z 324). The counts are plotted against the time in minutes.
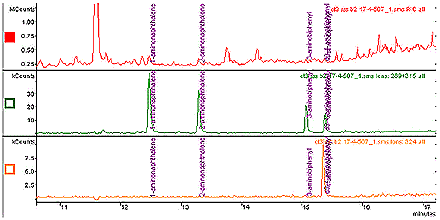
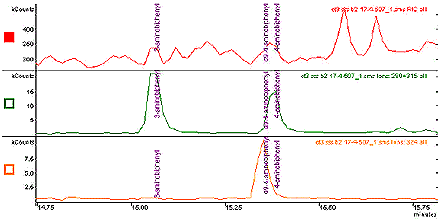
Figure 2: Expanded view of Figure 1, showing resolution of D9-4-aminobiphenyl and 4- aminobiphenyl.
The following image displays an expanded view of figure 1, showing a resolution of D9-4-aminobiphenyl and 4-aminobiphenyl by zooming into time 15-15.75 minutes in the x-axis.