Determination of Ammonia in Sidestream Tobacco Smoke
Health Canada
T-201, December 31, 1999
Table of Contents
- Scope of Applications
- Normative References
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- References
- Appendix
1 Scope of Applications
- Applicable to the isolation and quantitation of ammonia in sidestream tobacco smoke.
2 Normative References
- American Society for Testing and Materials (ASTM) D1193-77 - Standard Specification for Reagent Water, Version 1977.
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- Two cigarettes* are smoked beneath a fishtail chamber, with a 44 mm Glass Fibre Filter Disc (pad) and one impinger. The sidestream smoke is swept up the fishtail chamber at a rate of 3 L/minute by attenuated vacuum pressure.
*For other tobacco products, select a number such that breakthrough does not occur.
Note: The adequacy of a single impinger must first be evaluated by creation of a smoke train with three impingers in series. All components of the smoke train must be analyzed separately in order to determine trapping efficiencies. If breakthrough to the second and third impinger is greater than 3 % of the first impinger, these must be included in the smoke train. Trapping efficiencies may differ slightly dependent on impinger design. - At the completion of smoking, the fishtail is rinsed with the 0.1N H2SO4 solution. This solution is then used to extract the sidestream filter pad that has been folded into quarters and placed into a 250 mL Erlenmeyer flask. The flask is sealed and placed on a wrist action shaker for extraction.
- The pad is extracted on a wrist action shaker for 30 minutes. This mixture is then filtered through a 0.45 μm syringe filter into a scintillation vial where the sample may be stored at 4 °C for up to 48 hours.
- An aliquot of the filtered sample is then diluted with 0.025N H2SO4 in a 1:10 ratio, in order to achieve a 0.025 N concentration of H2SO4 in the sample for analysis. This solution is then transferred to an autosampler vial and analysed by cation exchange chromatography.
- A 35 μL volume of sample is injected onto a cation exchange analytical column that uses a Carboxylic acid/Phosphonic acid functional group to achieve separation of ammonium and monovalent cations. In order to adequately resolve sodium from the ammonium cation for quantitation, a 0.003 N (Normal) methanesulfonic acid solution is used as the mobile phase. After the ammonium ion has eluted, a gradient using concentrations of 0.2N H2SO4 to a 0.5N H2SO4 is used to remove any divalent cations and quaternary amines that may be present in the sample.
- Detection of cations is achieved using a suppressed conductivity detector in external water mode. This method of detection reduces background conductivity from the mobile phase, thus increasing the sensitivity of the detector for the analyte.
- Quantitation is obtained from a five point external standard calibration using the peak height response of ammonium sulphate. The amount of ammonia per cigarette is determined by calculating the amount of ammonia present in the analytical solution, then multiplying by the appropriate multiplier (impinger volume X dilutions) and divisor (# of cigarettes).
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- Analytical balance measuring to at least 4 decimal places.
- Vacuum pumps - GAST or equivalent.
- Tweezers and gloves for transferring pads.
- 250 mL PMP (Polymethylpentene) Erlenmeyer flasks with screw top cap closure.
- Constant rate wrist-action shaker.
- Syringe filter - Glass Fibre (25 mm X 0.45 μm).
- 250 mL impingers without frits.
- 25, 50, and 100 mL volumetric flasks.
- Disposable 5 cc syringe.
- 7 mL screw top vials with aluminum lined cap.
- Autosampler vials, caps and Teflon faced septa.
- High Performance Liquid Chromatograph (HPLC) consisting of:
- Refrigerated autosampler with 100 μL partial fill loop.
- Tertiary gradient system.
- Column heater.
- Dionex ED-40 conductivity detector or equivalent.
- Dionex CTC-1 cation trap or equivalent.
- Dionex CSRS-II conductivity suppresser in external water mode or equivalent.
- Column heater and temperature controler.
- Dionex IonPac CS12A cation exchange analytical column (250 mm X 4 mm) or equivalent.
- Dionex IonPac CG12A cation exchange guard column (50 mm X 4 mm).
- Data collection system.
- Fishtail chimney.
- Flow meter.
6 Reagents and Supplies
Note: All reagents shall be at the least, recognized as analytical reagent grade in quality.
- Ammonium Sulphate 99 % purity.
- Sulphuric Acid 96 % w/w.
- Methanesulphonic Acid (MSA) 100 % purity.
- Type I water (meets ASTM D 1193 specification).
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
- Immediately prior to use, all impingers are rinsed two times with 0.1N H2SO4, then three times with Type I water.
8 Preparation of Solutions
- Sulphuric Acid, 0.10N - Impinger Solution
- Carefully add 5.108 g of H2SO4 to 900 mL of Type I water.
- Mix and dilute to 1 L with Type I water.
- Sulphuric Acid, 0.20N - Solution C (Ion Chromatography)
- Carefully add 10.216 g of H2SO4 to 900 mL of Type I water.
- Mix and dilute to 1 L with Type I water.
- MSA 0.003N - Solution A (Ion Chromatography)
- Carefully add 0.2883 g of Methanesulphonic Acid (MSA) to 900 mL of Type I water.
- Mix and dilute to 1 L with Type I water.
9 Preparation of Standards
- Primary (1°) Ammonium Stock:
- Accurately weigh 0.20 g of ammonium sulphate into a 50 mL volumetric flask.
- Dissolve in 0.10 N H2SO4.
- Mix and dilute to 50 mL with Type I water.
- Prepare fresh every 10 working days.
Note: This corresponds to a 1.0898 mg/mL NH4+ ion stock solution.
- Working Standards:
Note: All working standards are made to volume to have a 0.025 N H2SO4 concentration.Working Standards Standard # Volume of 1o (μL) Final Volume (mL) Concentration [μg/mL] 0 0 25 0.000 1 250 25 10.898 2 175 25 7.6283 3 75 25 3.2693 4 75 50 1.6346 5 50 100 0.5449 6 20 100 0.2180
Note: All weights, volumes, and purity must be recorded and used to accurately calculate the standard concentrations. Prepare fresh every five working days.
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be specified in T-115.
11 Tobacco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars shall be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 (with the following modifications):
- Prepare the impingers for the determination of sidestream (SS) ammonia by aliquoting 100 mL of 0.1N H2SO4 into the 250 mL impinger ("large").
- Place the impinger, with top, onto the rear section of the smoking machine. Tubing from the impinger internal stem connection is connected to the SS filter cassette holder, and the sidearm attaches to the vacuum source, drawing 3 L/minute.
The following figure demonstrates sidestream apparatus. The BAT fishtail chimney contains a mainstream pad which connects to the port of the smoking machine at its base, and the tygon tube connects the fishtail to the sidestream pad holder on the top of the chimney. The sidestream pad holder on the BAT fishtail chimney is connected by tygon tubing to the 250 mL impinger containing the extraction solution is then connected outwards by plastic tube connectors to a flow meter, and is also further connected to a pump that controls the flow rate of the exhaust.
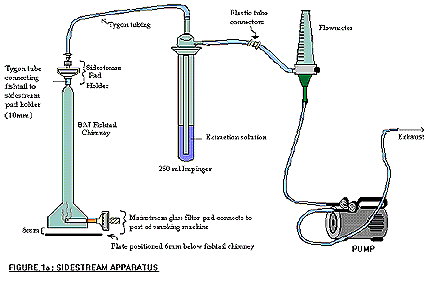
- Attach the weighted and numbered pad holders to the corresponding port/sidestream chamber configuration on the smoking machine.
- Anchor the chimney into the smoke machine chimney support. Raise the chimney level to the highest position (loading position).
- The machine conditions shall be as those specified in T-115 (with the following modifications):
13 Sample Generation
- Smoking is performed as specified in T-115 with the following modifications:
- Turn the vacuum source on just prior to lighting the cigarette.
- Light the cigarette and initiate the puff count.
- Lower the chimney to its lowest position.
Note: Do not allow the cigarette to touch the chimney. Keep the chimney approximately 6 mm from the plate insert.
- Burn the cigarette just to the line, remove the cigarette and extinguish the butt.
- Leave the pump on for approximately 30 seconds to allow the smoke to clear from the fishtail chimney.
- Turn the pump off, and raise the chimney to its highest position.
- Re-weigh the SS pad and holder and record the weight.
14 Sample Analysis
- Extraction of samples
- Transfer the impinger solution to a 250 mL Erlenmeyer flask.
- Rinse the impinger with 50 mL of fresh 0.1N H2SO4 and wash the fishtail chamber, collecting the rinsing in the same 250 mL Erlenmeyer flask.
- Repeat with another 50 mL of fresh 0.1 N H2SO4.
Note: Rinses must be done extremely carefully and thoroughly.
- All impinger and fishtail washes are mixed together in the 250 mL flask. The total volume of the extraction solution is 200 mL.
- Remove the pad from the sidestream holder and fold into quarters and place into the same 250 mL flask as the fishtail washings.
- Stopper and shake on wrist action shaker for 30 minutes.
- Sample Clean-up
- Filter the solution through a syringe filter into a 7 mL storage vial noting to rinse the vial initially with approximately 1 mL of sample.
- Quantitatively transfer 100 μL of the filtered extract to an autosampler vial.
- Quantitatively add 1000 μL of 0.025N H2SO4 to the same autosampler vial to dilute the sample and maintain approximately a 0.025 N H2SO4 concentration for analysis.
- Samples can be refrigerated for up to 48 hours prior to analysis.
Note: Additional dilutions may be necessary in order for the samples to be within the calibration range. In this case all dilutions should be made attempting to maintain a 0.025N H2SO4 concentration.
Note: Dilutions are not required to be performed in this manner. Dilutions using volumetric flasks may be more accurate (but more time consuming and susceptible to contamination).
- Instrument Analysis
- Dionex ED-40 Conditions
Suppressor Conductivity (SRS): 100 mA.
Scale: 20 μS.
Output: Offset.
Offset: 1 % of Full Scale. - Autosampler : Injection Volume
- Analyse using a 100 μL sample loop with the parameter for injection volume in the sample list at 35 μL with a 60 μL wash.
- Column Temperature: 30 °C.
- Mobile Phase / Gradient Conditions (Tertiary Gradient System)
Solvent A: 0.003N MSA.
Solvent B: Type I water.
Solvent C: 0.2N H2SO4.
Flow: 1.5 mL/minute.
Gradient: Minor adjustments may be required depending on column conditions and resolution of analyte.
Equilibration Time: 9.00 minutesTime (minutes) Composition % A % B % C 0.00 100 0 0 13.00 100 0 0 13.01 0 80 20 14.00 0 80 20 14.01 0 90 10 19.00 0 90 10 19.01 0 99 1 20.00 0 99 1 25.00 99 1 0 25.00 Method End Action: Equilibrate
- Dionex ED-40 Conditions
- Calculations
- Determination of Response Factor (RF)
- An initial calibration is performed by running prepared standards from high concentration to low concentration (injecting the very first standard minimum of two times until the response and retention time are constant).
- A calibration curve is prepared by plotting the concentration of NH4+ ion in the standard vs. the peak height response from the conductivity detector.
- The Response factor is the slope of the line as determined by linear regression (Height counts / unit concentration).
- Determination of Ammonium Ion
NH4+ [μg/cigarette] = (Peak Height × Volume Extractant (mL) × Final Volume (mL)) / (RF × # Cigarettes Smoked × Aliquot Volume (mL))
where the aliquot volume (mL) is the volume transferred to the autosampler vial. - Determination of Ammonia
NH3 [μg/cigarette] = NH4+ [μg/cig] × (17/18).
where 17/18 corrects for molecular weight. - Determination of Total Particulate Matter (TPM)
TPM [mg/cigarette] ={[Pad and holder after smoking (g) - Pad and holder before smoking (g) ] × 1000 mg/g} / # of cigarettes smoked
- Determination of Response Factor (RF)
15 Quality Control
- Typical Chromatogram
- See Appendix 1.
- Typical Control Parameters
- Laboratory Reagent Blank (LRB): Before processing any samples, the analyst should demonstrate, through the analysis of a reagent water blank, that interference from the analytical system, glassware, and reagents are not present.
- For each analytical batch, a LRB and laboratory fortified blank (LFB) must be analyzed. The blank and spiked samples must be carried through all stages of the sample preparation and measurement steps.
- A cigarette of known characteristics (e.g. Kentucky monitor 1R4F) should be included with each group of unknown samples.
- Laboratory Fortified Matrix (LFM): To assess matrix interference, spike a true sample with a known concentration and determine the % Recovery.
- Recoveries and Levels of Contamination
- Typical recoveries of Laboratory Fortified Blanks (LFB) and Laboratory Fortified Matrix (LFM) samples range from 85 - 110 % when a spiked solution (or sample) is carried out through the entire extraction process.
- Typical Laboratory Reagent Blanks (LRB) has a calculated value of 0μg/cigarette. Contamination of this type is usually associated with contamination of the filter pad during conditioning or an inadequate cleaning of glassware.
- Method Detection Limits (MDL) / Limit of Quantitation (LOQ)
- The method detection limit (MDL) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The MDL is calculated as three times the standard deviation of these determinations.
- The MDL (on a ng/cigarette basis) can be varied by modifying the number of cigarettes smoked and the volumes used for extraction and clean-up in the procedure.
- The practical limit of quantitation (LOQ) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The LOQ is calculated as 10 times the standard deviation of these determinations.
- For true samples, the MDL/LOQ is dependent on the resolution and the amount of sodium ion present in the sample, since the tail of a huge sodium peak may mask any ammonium ion present.
- Stability of Reagents and Samples
- Primary standards should be prepared fresh every 10 working days and be stored at 4 °C.
- Run standards should be prepared fresh from the stock solution weekly and be stored at 4 °C.
- Samples are stable at 4 °C for one week after extraction.
- Diluted Samples must be run within 48 hours.
16 References
- Risner, C.H., Conner, J.M. Collection of Ammonia in Indoor Air by Means of a Weak Cation Exchange Cartridge. Environmental Toxicology and Chemistry, Vol. 10, p. 1417-1423, 1991.
- Nanni, E.J., Lovette, M.e., Hicks, R.D., Fowler, K.W. and Borgerding, M.F. Separation and Quantitation of Monovalent and Cationic Species in mainstream Cigarette Smoke Aerosols by High-Performance Ion Chromatography. Journal of Chromatographic Science, Vol. 28, August 1990.
- IonPac CS12A Analytical Column, Installation Instructions and Troubleshooting Guide, Document No. 031132, Revision 01, Dionex Corporation, 1995.
17 Appendix
Appendix 1: Typical Chromatogram
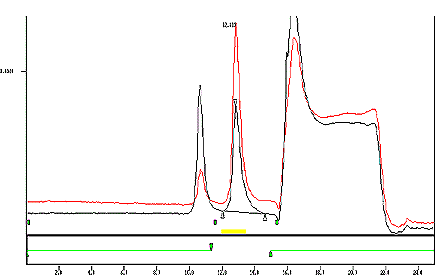
An overlay of a standard and a Reference cigarette with a 5 % offset.