Determination of Benzo [A] Pyrene in Sidestream Tobacco Smoke
Health Canada
T-203, December 31, 1999
Table of Contents
- Scope of Applications
- Normative References
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- References
- Appendices
1 Scope of Applications
- Applicable to the quantitation of Benzo[a]pyrene B[a]P content in total particulate matter (TPM) of sidestream (SS) tobacco smoke by reversed phase high performance liquid chromatography (HPLC) via fluorescence detection.
2 Normative References
- American Society for Testing and Materials (ASTM) D1193 -77 Standard Specification for Reagent Water, Version 1977.
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
- Methods of Sampling and Testing Tobacco - Determination of Benzo[a]pyrene in Total Particulate Matter of Tobacco Smoke. National Standard of Canada, Canadian General Standards Board CAN/CGSB-176.2 No. 1-96, March 1996.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- Cigarettes are smoked beneath a British American Tobacco (BAT) fishtail chamber with a 3 L/minute flow rate in order to capture the sidestream smoke.
- Total particulate matter (TPM) of the sidestream smoke, collected on a glass fibre filter disc (pad), is extracted with a sufficient amount of acetone to create a homogeneous solution of tar in acetone. A portion of this solution is filtered through a 0.45 μm syringe filter to a 7 mL glass vial for storage.
- A 2 mL aliquot of this extract is evaporated under a constant stream of nitrogen to dryness and reconstituted in 2 mL of cyclohexane. This cyclohexane solution is passed through a 1 g (6 mL) silica cartridge and 360 mg NH2 plus cartridge mounted in series. The B[a]P is eluted with hexane, evaporated to dryness, and made to a 1 mL volume with acetonitrile.
- The sample is subjected to reversed phase liquid chromatography and quantitated via fluorescence detection.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- Supelco Visi-Prep Solid Phase Extraction unit (24 cartridge unit) or equivalent.
- 2 mL glass pipettes.
- Brinkman Dispensette (10-50 mL) or equivalent.
- Micro-pipettes (5, 1000 µL).
- 16 X 125 mm culture tubes (20 mL).
- 125 mL Erlenmeyer flasks with ground glass joints or PMP Erlenmeyers with screw top caps.
- Zymark turbo-vap or equivalent.
- GAST Pumps or equivalent.
- Flowmeters.
- BAT Fishtail Chambers.
- Glass fibre filter discs (pads) and holders (45 mm).
- Disposable 5 cc syringe.
- Auto sampler vials, caps and septa.
- Pasteur Pipettes.
- 1 g silica Sep-Pak cartridges (6 mL capacity).
- 360 mg NH2 Plus Sep-Pak cartridge.
- 7 mL screw top vials with aluminum lined cap.
- 0.45 μm glass fibre syringe filters 25 mm.
- Merck 250 X 4 mm, RP-18e, 5 μm packing, HPLC column with a Lichrocart 4-4 Lichrosphere 100 RP-18 endcapped, 5 μm guard column or equivalent.
- High Performance Liquid Chromatograph with:
- Fluorescence detector.
- Autosampler.
- Tertiary pump.
- Data collection system.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade quality.
- Benzo[a]pyrene (B[a]P).
- Cyclohexane.
- Hexane.
- Acetonitrile.
- Methanol.
- Isopropanol (IPA).
- Anhydrous Sodium Sulfate.
- Acetone.
- Tetrahydrofuran (THF).
- Type I water (meets ASTM D 1193 specifications).
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
8 Preparation of Solutions
- Prepare solutions required for analysis, as specified in T-115, in accordance with good laboratory practice.
9 Preparation of Standards
- Preparation of Spiking Solution for Laboratory Fortified Samples
- Primary B[a]P Stock: Dissolve 10 mg B[a]P solid to 50 mL Cyclohexane.
- Secondary Stock: Pipette 50 μL of 1o Stock to 50 mL Cyclohexane.
- A 10 μL volume of spiking solution is added to a second 2 mL aliquot of a control brand cigarette extract solution prior to solvent substitution and clean-up through the SPE cartridges (LFM*). Another 10 μL volume of spiking solution is added to a second 2 mL aliquot of the LRB* prior to solvent substitution and clean-up through the SPE cartridges (LFB*). The spiking analytical concentration is approximately 2 ng/mL (dependent on stock concentration).
*See section on Quality Control for explanations of these initialisms.
- Preparation of Working Standards
- Primary (1°) B[a]P Standard: Dissolve 10 mg B[a]P to 50 mL Acetonitrile.
- Secondary (2°) Standard: Pipette 100 μl of 1o Standard to 50 mL Acetonitrile.
- Working Standards:
Working Standards Standard # Volume of (2o) Standard (μL) Final Volume (mL) Concentration [ng/mL] 1 40 25 0.6400 2 175 25 2.800 3 350 25 5.600 4 600 25 9.600 5 900 25 14.4 6 2 mL of Std # 1 10 0.1280 7 4 mL of Std # 1 10 0.2560 - All weights, volumes, and purity must be recorded and used to accurately calculate the standard concentrations. These concentrations are only representations of standards used in a calibration curve.
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product is to be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks, and cigars are to be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 with the following modifications as detailed below:
- The B[a]P sidestream apparatus (smoke train) is assembled as per diagram.
- The flowmeters are calibrated so that a measured flow rate of 3 L/minute could be monitored with the sidestream filter pad present. Calibrate all ports recording the flowmeter setting.
- Weigh conditioned cigarettes (three cigarettes* / observation) and the sidestream filter holder prior to smoking.
*For other tobacco products, select a number such that breakthrough does not occur. - Place the conditioned cigarettes in position using the calibrated ports.
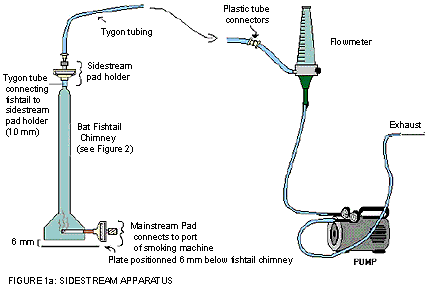
The following figure demonstrates sidestream apparatus. The BAT fishtail chimney contains a mainstream pad which connects to the port of the smoking machine at its base; whereas the tygon tube is connecting the fishtail to the sidestream pad holder on the top of the chimney. The sidestream pad holder on the BAT fishtail chimney is connected to the flow meter which is further connected to a pump that controls the flow rate of the exhaust.
- The machine conditions shall be as those specified in T-115 with the following modifications as detailed below:
13 Sample Generation
- Turn on the sidestream pumps 30 seconds prior to the first puff taken by the smoking machine.
- Light the cigarette on the first puff with the cigarette below the fishtail.
Note: Special consideration of lighting procedures for specific types of cigarettes must be followed.
- Lower the fishtail directly over the cigarette positioning a plate 6 mm below the fishtail to create a uniform air flow up the chimney.
- When the cigarette is smoked to the end mark, completely remove the butt from the chamber as to no longer contribute any more sidestream smoke. Turn off the pump 30 seconds after the cigarette has been extinguished in order to pull all smoke present in the chimney through the sidestream filter pad.
- Repeat steps 13.1 through 13.4 with the second and third cigarette.
- After the smoking is complete, disassemble the sidestream apparatus and weigh the sidestream filter holder to determine TPM.
- Record weights of TPM for sidestream pads.
14 Sample Analysis
- Extraction of Sidestream Pads
- Remove the sidestream pad from its holder, folding it into quarters and wiping the inside of the holder with the clean side of the pad.
- Add this sidestream pad to a 125 mL Erlenmeyer flask and cap.
- Rinse the fishtail chimney with a volume of acetone, such that the final concentration of TPM in the flask is approximately 1 mg/mL, collecting the rinse in the 125 mL Erlenmeyer flask containing the pad.
For example: The volume of acetone used to rinse the fishtail is numerically equivalent to the total TPM in mg (rounded to the nearest 10 mL) yielding a concentration of approximately 1 mg TPM/mL of acetone.
Example: 1. If TPM = 70 mg (total), then acetone volume = 70 mL.
Example: 2. If TPM = 83 mg (total), then acetone volume = 80 mL.
Example: 3. If TPM = 57 mg (total), then acetone volume = 60 mL.
Note : The maximum amount of acetone to be added is 100 mL.
- Record the volume of acetone used to rinse the fishtail and subsequently extract the pad.
- Shake the Erlenmeyer containing the sidestream pad and acetone vigorously on a wrist action shaker for 30 minutes, until there appears to be a homogeneous solution and there is no longer any evidence of localized colour on the pad.
- Place the flasks in the dark to allow some of the broken-up pad to settle.
- Sample Clean-up
- Filter approximately an 8 mL portion of the acetone extract through a 0.45 μm disposable filter into a 7 mL vial with foil lined cap (samples may be stored at 4 °C at this point).
- Pipette a 2 mL aliquot of the acetone extract to a 16 X 125 mm culture tube.
- Place the tubes containing the 2 mL sample into the turbo evaporator.
Note: Turbo-vap conditions are to be set at 40 oC with a Nitrogen pressure of 7.5 psi.
- Evaporate the samples to complete dryness.
- Redissolve the sample by pipetting 2 mL of cyclohexane to the tube and vortex for 15 seconds (vortex each sample twice).
- Pre-condition both (the silica and the NH2 plus) cartidges using hexane as recommended by manufacturer.
Note: All air has to be removed from the packing thus exposing sorbent material to solution.
- Place the pre-conditioned cartridges on the Visi-prep unit, add approximately 1 g anhydrous sodium sulfate to the silica cartridge, and wash the cartridges with 10 mL hexane allowing the hexane to flow through the cartridge by gravity.
- Transfer the 2 mL cyclohexane solution onto the packing of the Silica cartridge.
Note: High tar samples containing high amounts of B[a]P may require only 250-500 μL to be transferred. - Allow the cyclohexane to pass through the SPE cartridges (by gravity) at a rate of approximately one drop/second. Discard the eluant.
- Pipette 4 mL hexane to the cartridge allowing the eluant to gravimetrically pass through the cartridge. Discard the eluant.
Note: If the entire sample has been transferred, use these volumes of hexane to further wash the culture tube to ensure the sample has been quantitatively transferred.
Note: Elution volumes must initially be determined with the use of a different manufacturer's cartridge and should be checked between lot numbers.
- Place 20 mL disposable glass culture tubes beneath each of the cartridges.
- Gravimetrically elute the B[a]P from the cartridges with 4 X 4 mL additions of hexane.
- Add 1 mL of THF to each tube.
- Place the tubes containing the 17 mL of collected eluant into the Zymark Turbo-vap.
Note: Turbo-evaporation conditions are to be set at 40 oC with a Nitrogen pressure of 7.5 psi.
- Evaporate the samples to complete dryness.
Note: This will require an initial 20 minutes of evaporation in which the nitrogen pressure may be slowly increase to a maximum of 10 psi in such a manner as to prevent any loss of sample from splattering.
- Remove samples that are completely dry. If some samples are not completely dry, evaporate the samples in additional five minutes intervals.
- Pipette 1000 μL of Acetonitrile into each of the dried tubes to dissolve the analyte and any residue that may be present.
- Vortex the sample at high speed for approximately 15 seconds.
- Using a glass transfer pipette, wash down the sides of the tube five times with the sample, and transfer the sample to a 2 mL autosampler vial with a screw cap and Teflon faced septa.
- The samples are ready for HPLC analysis and may be stored at 4 °C until they are analyzed.
- Instrument Analysis: Reversed Phase High Performance Liquid Chromatography (HPLC) Analysis
- Jasco Fluorescence Detector Conditions
Excitation Wavelength: 365 nm.
Emission Wavelength: 425 nm.
Gain: X 1000.
Attenuation: 32.
Note: A different manufacturer's fluorescence detector may need to be programmed differently to maintain the full calibration range. A slight change in excitation and emmision wavelength may be required dependent on manufacturer (i.e. 366 and 424 for the wavelengths).
- Autosampler : Injection Volume
- Analyze using a 50 µL sample loop with the parameter for injection volume in the sample list at 75 μL to ensure a thorough flushing of the sample loop with the sample.
- Mobile Phase / Gradient Conditions (Tertiary Gradient System)
Solvent A: 55: 45 Acetonitrile: 1 % IPA in Type I water (degassed and filtered with a 0.45 μm nylon filter).
Solvent B: Methanol.
Solvent C: Acetonitrile.
Flow: 1.5 mL/minute.
Gradient: Adjustments to the gradient may be required depending on column conditions and resolution of analyte.
Equilibration Time: 8.00 minutes.Time (minutes) Composition % A % B % C 0.00 55 0 45 20.00 75 0 25 25.00 100 0 0 28.00 100 0 0 30.00 0 100 0 32.00 0 100 0 34.00 100 0 0 35.00 100 0 0 35.00 Method End Action: Equilibrate
- Jasco Fluorescence Detector Conditions
- Calculations
- Determination of Response Factor (RF)
- An initial calibration is performed by running prepared standards from high concentration to low concentration (injecting the very first standard a minimum of two times until the response and retention time are constant).
- A calibration curve is prepared by plotting the concentration of B[a]P in the standard vs. the peak height response from the fluorescence detector.
- The Response factor is the slope of the line as determined by linear regression (Height counts / unit concentration).
- Determination of Response Factor (RF)
- Determination of B[a]P Delivery [ng/cig]
- B[a]P [ng/cig] = [Peak Height × Volume Extractant (mL) × Final Volume (mL)] /
[RF × # Cigarettes Smoked × Aliquot Volume (mL)]
where the aliquot volume (mL) is the volume transferred to the Sep-pak cartridge correcting for any potential previous dilutions in the solvent substitution step. The Response Factor is to be determined from the calibration curve.
- B[a]P [ng/cig] = [Peak Height × Volume Extractant (mL) × Final Volume (mL)] /
[RF × # Cigarettes Smoked × Aliquot Volume (mL)]
15 Quality Control
- Typical Chromatogram
- See Appendix 1a and 1b.
- Typical Control Parameters
- Each set of analysis should contain at least one of each of the following per day of smoking or batch of up to 16 samples (four sets of four samples).
- Laboratory Reagent Blank (LRB): To determine background contamination from solutions, glassware, or materials used in the analysis process.
- Laboratory Fortified Blank (LFB): To determine whether there is any loss of analyte as a result of the analysis process.
- Laboratory Fortified Matrix (LFM): By spiking of the control brand cigarettes: To determine whether there is any loss of analyte as a result of the analysis process and to determine potential matrix effects.
- Reference Sample: To determine the inter-experimental reproducibility of the entire method of analysis.
- Duplicate Sample: To determine the reproducibility of the procedure within the same experiment or batch on analysis.
- Each set of analysis should contain at least one of each of the following per day of smoking or batch of up to 16 samples (four sets of four samples).
- Recoveries and Levels of Contamination
- Typical recoveries of Laboratory Fortified Blanks (LFB) and Laboratory Fortified Matrix (LFM) samples range from 85 - 110 % when a spiked solution (or sample) is carried out through the entire extraction process.
- Recoveries lower than 85 % indicate either an insufficient elution of B[a]P from the solid phase extraction cartridges or a change in response factor (RF) of the fluorescence detector. A change in RF must first be investigated before re-processing of samples is initiated.
- Typical Laboratory Reagent Blanks (LRB) range from a calculated value of 0 - 0.3 ng/cigarette. Contamination of this type is usually associated with contamination of the filter pad during conditioning or an inadequate cleaning of glassware.
- Method Detection Limits (MDL) / Limit of Quantitation (LOQ)
- The method detection limit (MDL) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The MDL is calculated as three times the standard deviation of these determinations.
- The MDL (on a ng/cig basis) can be modified by varying the number of cigarettes smoked and the volumes used for extraction and clean-up in the procedure.
- The practical limit of quantitation (LOQ) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The LOQ is calculated as 10 times the standard deviation of these determinations.
- Stability of Reagents and Samples
- Analytical stocks and standards should be stored at -20 °C.
- Stock standards and stock spike solutions remain stable for up to six months. Although there is no loss of analyte, evaporation (loss) of solvent may be an issue.
- Analytical run standards should be freshly prepared every two months.
- Samples are stable at 4 °C for three weeks after extraction.
16 References
- Proctor, C.J., Martin, C., Beven, J.L., and Dymond H.F., 1988. Evaluation of an Apparatus Designed for the Collection of Sidestream Tobacco Smoke, Analyst 113: p. 1509-1513.
- Dumont, J., Larocque-Lazure, F., and Iorio, C., 1993. An Alternative Isolation Procedure for the Subsequent Determination of Benzo[a]pyrene in Total Particulate Matter of Cigarette Smoke. Journal of Chromatographic Science, Vol. 31. September 1993. p. 371-374.
17 Appendices
Appendix 1a : Typical Chromatogram
The following image displays a typical chromatogram with an overlay of a high tar reference cigarette and a low tar reference cigarette, with a peak at 14.923 minutes.
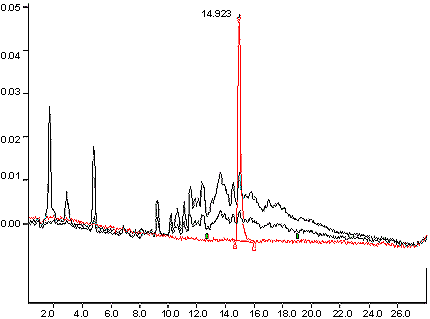
An overlay of a standard, a high tar reference cigarette and a low tar reference cigarette.
Appendix 1b : Typical Chromatogram
The following image displays an expanded view of appendix 1a to show the integrating baseline of a true sample.
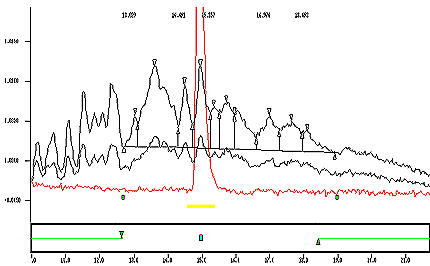
An expanded view of Appendix 1a to show the integrating baseline of a true sample.