Determination of Mercury in Sidestream Tobacco Smoke
Health Canada
T–206, December 31, 1999
Table of Contents
- Scope of Applications
- Normative References
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Solutions and Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- References
- Appendix
1 Scope of Applications
- This method is to be used to determine the amount of Hg in sidestream tobacco smoke. The method is designed to trap and quantitate both the particulate phase and gaseous phase components of the smoke together in the same impinger solution as it is smoked on a linear smoking machine.
- Sidestream smoke is, effectively, all the smoke emitted from a cigarette other than the mainstream smoke. This is collected using a glass fishtail chimney assembly to direct the smoke to various trapping devices.
- Particulate phase mercury can not be separated from gaseous phase mercury using this type of trapping and analysis system.
- Mercury compounds are analysed by automated cold vapour atomic absorption spectroscopy : EPA Stannous Chloride Methodology
2 Normative References
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
- American Society for Testing and Materials (ASTM) - D1193-77 Standard Specifications for Reagent Water, Version 1977.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- Equidistant ports of a standard linear smoking machine are reconfigured with the BAT (British American Tobacco) fishtail chambers and flow controlled vacuum pumps. Five cigarettes* per port are smoked beneath the fishtail chambers. The smoke is swept up the chimney at the rate of 2 L/minute.
*For other tobacco products, select a number such that breakthrough does not occur. - The analyte is collected by passing the whole tobacco smoke through two impingers in series containing an acidified potassium permanganate solution. The particulate phase trapped on the wall of the fishtail is washed off using 2 X 5 mL additions of acidified potassium permanganate solution, 1 X 5 mL addition of hydrogen peroxide and 1 X 5 mL addition of Type I water. This rinse is added to a microwave digestion vessel already containing the two impinger solutions combined. The samples are then subjected to microwave digestion.
- When digestion is complete, the vessels are removed from the digestor, allowed to cool. Excess potassium permanganate is reduced with hydroxyamine hydrochloride, and transferred to a volumetric flask where they are made to volume with Type I water.
- The digestate is analysed by using cold vapour atomic absorption spectroscopy at 253.7 nm. This method uses a continuous flow vapour generator to reduce the divalent mercury to its atomic state with stannous chloride. A peristaltic pump pushes the reducing agent and sample through a mixing coil to a gas liquid separator. Nitrogen gas carries the mercury vapour into a flow cell positioned in the burner compartment.
Note: The reaction is very sensitive to fluctuations in temperature so the response must be checked frequently against standards.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- 70 mL impingers without frits.
- 1/4" ID X 3/8" OD ester grade Tygon tubing.
- 1/4" Nalgene connectors.
- 44 mm glass fibre filter discs (pads) and cassettes.
- 20 X 150 mm disposable borosilicate culture tubes.
- Linear Smoking Machine - Filtrona SM300 20 port smoking machine or equivalent.
- Vacuum Pumps - GAST (4) or equivalent.
- Flowmeters.
- Fishtail Chambers - BAT (8).
- Analytical Balance measuring to at least four decimal places.
- Mini Hot Plate / Stirrer.
- 50 mL, 100 mL, 1000 mL volumetric flasks.
- Eppendorf or micro-pipettes for the preparation of analytical run standards.
- Eppendorf pipette (1-5 mL adjustable volume) or equivalent.
- 125 mL HDPE storage bottles.
- Varian 400P Atomic Absorption Spectrophotometer or equivalent.
- Varian PSC-56 Programmable Sample Changer or equivalent.
- Varian VGA-76 Vapour Generation Assembly or equivalent.
- Varian Mercury Flow Through Cell or equivalent.
- Hollow Cathode Lamp for Hg.
- CEM MDS-2100 Microwave Digestion System or equivalent.
- CEM ACV-12 Digestion Vessel Assembly (X 2) or equivalent.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical grade in quality.
- Concentrated hydrochloric acid (HCl).
- Concentrated sulphuric acid (H2SO4).
- Concentrated nitric acid (HNO3).
- Type I water (meets ASTM D 1193 specifications).
- Potassium Permanganate (KMnO4).
- Hydrogen peroxide (H2O2) 30-32 %.
- Stannous Chloride.
- Hydroxylamine Hydrochloride.
- Atomic Absorption Reference Standards - Mercury standard solution at 1000 μg/mL in 10 % HNO3.
Note: Reference standards must:- Come with a certificate of analysis.
- Be NIST traceable.
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
Important: The cleaning of glassware and the cleanliness of the environment in which the analysis is performed, has a direct effect on the accuracy and precision of the method. In order to achieve accurate results, all glassware must be cleaned immediately prior to use with dilute HCl (1+1) and then rinsed with Type I water.
8 Preparation of Solutions
- Sulphuric Acid / Potassium Permanganate Impinger Solution (20 % H2SO4 v/v, 4 % KMnO4 w/v )
- Add approximately 500 mL of Type I water to a 1000 mL volumetric flask.
- Carefully add 200 mL of concentrated H2SO4 to the flask and gently swirl and allow the flask to cool completely to room temperature before proceeding.
- Add 40 g of potassium permanganate to the flask and continue to mix until it appears that all the permanganate is dissolved.
- Make solution to volume with Type I water.
Note: When diluting a concentrated acid solution, it is always important to add the acid to water.
Note: This solution is stable for a maximum of one day due to potential contamination and precipitation of the permanganate.
- Hydroxylamine Hydrochloride Solution (10 % w/v )
- Add 10 g of hydroxylamine hydrochloride to a 100 mL volumetric flask.
- Add approximately 70 mL of Type I water to dissolve the solid.
- Make solution to volume with Type I water.
- Stannous Chloride Solution (25 % w/v SnCl2 in 20 % v/v HCl)
- Weigh 125 g of Stannous Chloride into an acid washed 500 mL volumetric flask.
- Add 100 mL of concentrated HCl to completely dissolve the solid material.
Note: Gentle heating may be applied in order to speed up this process. - Allow the solution to cool before carefully adding Type I water to make to the 500 mL volume.
- Mix well and transfer the contents to the 500 mL bottle for the reducing agent channel of the Vapour Generation Assembly.
Note: If any precipitate appears in the bottle or flask, discard the solution and prepare fresh. It is necessary to keep the stannous chloride in solution as well as contaminant free as possible.
9 Preparation of Solutions and Standards
- Analytical Standards Stocks and Required Dilutions
- All analytical standards are made to a 12 % (v/v) H2SO4 acid solution immediately prior to analysis, and are to be considered stable for only two days.
- The purchased stock standard is in a 10 % (v/v) HNO3 acid solution at a concentration of 1000 μg/mL for stability purposes.
- In order to make the proper dilutions, it is necessary to prepare a secondary stock standard at a concentration of 1 μg/mL also in a 10 % (v/v) HNO3 acid solution. This secondary stock solution is considered to be stable for one week.
- Representative dilutions are as follows:
Primary Stock = 1000 μg/mL.
Secondary Stock = 100 μL of Primary Stock to 100 mL = 1 μg/mL.
Standard Concentration = 0.300 ng/mL = 30 μL Secondary Stock to 100 mL.
Standard Concentration = 0.500 ng/mL = 50 μL Secondary Stock to 100 mL.
Standard Concentration = 1.500 ng/mL = 150 μL Secondary Stock to 100 mL.
Standard Concentration = 3.000 ng/mL = 300 μL Secondary Stock to 100 mL.
Standard Concentration = 5.000 ng/mL = 500 μL Secondary Stock to 100 mL.
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars shall be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 (with the following modifications as detailed below):
- Assemble the sidestream smoke train such that the smoke of cigarettes smoked beneath the fishtail chambers is drawn up the chimney a rate of 2 L/minute. The whole sidestream smoke is bubbled through two impingers each containing 25 mL of impinger solution (20 % H2SO4 v/v, 4 % KMnO4 w/v). Any particles (or acid) that pass through the impinger, are then trapped onto a standard glass fibre filter pad and holder before it goes through the flowmeter to the vacuum pump. This filter pad is not used in the analysis, but is only there to protect the vacuum pump from permanganate particles. See Diagram.
The following figure demonstrates sidestream apparatus. The BAT fishtail chimney contains a mainstream glass filter pad which connects to the port of the smoking machine at its base; whereas the tygon tube is connecting the fishtail to the sidestream pad holder on the top of the chimney. The sidestream pad holder on the BAT fishtail chimney is connected by tygon tubing to two 70 mL impingers in series, each containing the extraction solution. In addition the filter pads and holders are connected to a second impinger on one end and the flowmeter to the other. The flow meter is also further connected to a pump that controls the flow rate of the exhaust.
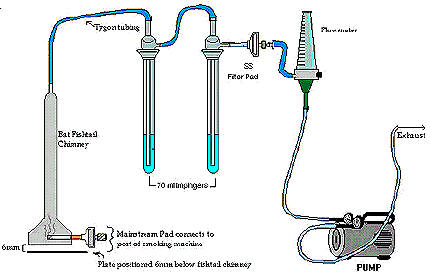
- The sidestream impingers are loaded with 25 mL of impinger solution (20 % H2SO4 v/v, 4 % KMnO4 w/v).
- Calibrate the vacuum pumps to draw at the rate of 2 L/minute. Record the flowmeter settings.
- Connect two clean 70 mL impingers, in series, containing 25 mL of fresh impinger solution (20 % H2SO4 v/v, 4 % KMnO4 w/v).
- Place a new filter pad into the back-up holder in line between the second impinger and the flowmeter.
13 Sample Generation
- Install the first test cigarette to be smoked in position below the fishtail of the calibrated ports.
- Turn on the sidestream pumps and begin the lighting procedure at 30 seconds prior to lighting the cigarette.
- Light the cigarette on the first puff and then lower the fishtail assembly over the cigarette to a position of 6 mm above a plate that is beneath the cigarette. This is to create a uniform flow of air around the cigarette and up the fishtail chimney.
- Smoking is terminated and the butt is extinguished and removed when the cigarette has been consumed to the predetermined end mark.
- The pump continues for an additional 30 seconds to sweep any residual smoke up to the sidestream filter.
- The smoking process is repeated for the second through fifth cigarette.
Note: When smoking under intense smoking conditions, the mainstream filter pad must be replaced after three cigarettes to prevent breakthrough. - At the end of the smoking process, disassemble the sidestream apparatus.
14 Sample Analysis
- Using positive pressure backwash the Tygon tubing for each of the impingers with the impinger solution.
- Both impinger solutions are transferred to the same digestion vessel.
- The particulate matter trapped on the wall of the fishtail is washed using 2 X 5 mL additions of fresh impinger solution.
- This rinse is transferred to the second of the two impingers for rinsing.
- This rinse is then transferred to the first impinger for rinsing and then carefully added to the digestion vessel containing the original impinger solutions.
- The fishtail is then rinsed using 1 X 5 mL of H2O2 that is transferred to the second of the two impingers for rinsing.
- This rinse is then transferred to the first impinger for rinsing and then carefully added to the digestion vessel containing the original impinger solutions.
- This rinsing process (14.1.6 to 14.1.7) is then repeated using a 1 X 5 mL of Type I water.
- Install the rupture membrane and cap the digestion vessel.
- Place the digestion vessel into the turntable and lock into position.
- Choose the sample that appears to be the most reactive sample as the reference vessel for monitoring pressure and temperature to control the digestion.
- Load the turntable of samples into the microwave digestor, and start the digestion program. See Appendix: Microwave Digestion Parameters.
- When the digestion is complete, remove the turntable from the microwave and allow the samples to cool to room temperature before opening.
- Add 5 mL of the hydroxylamine hydrochloride solution dropwise to react with the excess permanganate in the samples.
Note: If the digestion appears to be incomplete, by evidence of particulate matter in the digestate, carefully add one to two more mL of fresh impinger solution and/or hydrogen peroxide and repeat the original digestion procedure. - When the digestion is complete, remove the turntable from the microwave and allow the samples to cool to room temperature before opening.
- Transfer the digestate to a 100 mL volumetric flask and make to volume using the washing of the digestion vessel with Type I water.
Note: Samples must be analyzed within 48 hours of completing the digestion (24 hours recommended) and taking to volume for stability purposes. Manual dilutions (if necessary) of the digestate should only take place at the time of analysis. - Sample Dilutions Required for Elemental Analysis
- No further dilutions of the sample are required, and may be analysed as is.
- A portion of the diluted sample is transferred to a 20 mm X 150 mm disposable borosilicate culture tubes for analysis. The remainder of the solution is stored in the 125 mL HDPE storage bottle to prevent possible contamination.
Note 1: Any dilutions (if necessary) must be accounted for when calculating the results in a ng/cigarette basis.
Note 2: Sample volumes are based on "average" literature values. These dilutions may need to be modified depending on: 1. The samples country of origin, 2. The year in which the sample was grown (environmental factors), 3. The soil type and conditions which the sample was grown, 4. The type of tobacco used for the sample, 5. The stalk position of the tobacco used for analysis (if not a blended, finished product).
- Analysis of Hg by Cold Vapour Atomic Absorption
- Samples are analysed using the parameters established for the instrument at a wavelength of 253.7 nm and slit width of 0.5 nm.
- It is important to analyse the samples for Hg within 48 hours of completing the digestion.
- If samples are not analysed within this time frame, the digestate should be returned to the digestion vessel and the secondary digestion procedure performed.
Note: Parameters may slightly differ between instruments.
- Calculations
- Results reported by the computer controlled software are expressed as [ng/mL] in solution. This result, multiplied by the dilution of the sample and divided by the number of cigarettes smoked, will calculate the result in a [ng/cigarette] basis.
- The [ng/cigarette] results can be converted to [μg/cigarette] by dividing this result by 1000.
Analytical Result (on a "per cigarette" basis):
Analyte [ng/cigarette] = (Analytical result [ng/mL] × 100 mL × Additional Dilution factor) / No. of Cigarettes (5).
15 Quality Control
- Each set analysis should contain one of each of the following per day of smoking or batch of up to 24 analysis (20-22 true samples):
- Laboratory Reagent Blank (LRB): to determine background contamination from solutions or glassware used in the analysis process.
- Laboratory Fortified Blank (LFB): to determine whether there is any loss of analyte as a result of the analysis process.
- Reference Sample: to determine the inter-experimental reproducibility of the entire method of analysis
- Duplicate Sample: to determine the reproducibility of the procedure within the same experiment or batch on analysis.
Note: As an initial evaluation of the method and materials used, it is recommended that a minimum of five blanks be analysed using the method in order to establish control parameters for expected levels of background contamination before any samples are analysed. An LRB outside these control limits is an indicator of possible contamination problems or the use of materials and reagents of different lot numbers.
Note: Room contamination should also be analyzed by analyzing the background as a sample with no smoking occuring. This should be done once per day. If background is too high (most likely due to the existence of broken Hg thermometers), the entire room must be scrubbed down and filters changed in the ventillation system to remove existing contaminated dust. If a high background remains, the cleaning procedure must be repeated. - Recoveries and Levels of Contamination
- Contamination must be monitored with each individual set of samples that are digested and is dependent on the laboratory environment. This ultimately effects the precision of the analysis.
- Method Detection Limit (MDL) / Limit of Quantitation (LOQ)
Note: Individual instruments will have different MDL's and LOQ's depending on the optimization of the instrument.
The MDL is defined as either:- The concentration of analyte that yields an absorbance of 0.004 units (the characteristic mass); or
- Determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The MDL is calculated as three times the standard deviation of these determinations; or
- Same as number two using a blank solution.
The MDL (on a ng/cigarette basis) can be calculated by multiplying the determined MDL (ng/mL basis) by the final volume and dividing this by the number of cigarettes smoked.
The MDL (on a ng/cigarette basis) can be enhanced by varying the amount of cigarettes smoked, however this may affect the amount of background contamination observed.
The practical limit of quantitation (LOQ) may be defined as either:
- The lowest level of standard other than a blank used to construct a calibration curve; or
- Determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The LOQ is calculated as 10 times the standard deviation of these determinations; or
- Same as number two using a blank solution.
The LOQ (on a ng/cigarette basis) can be calculated by multiplying the determined LOQ (ng/mL basis) by the final volume and dividing this by the number of cigarettes smoked.
The effect of modifying the number of cigarettes smoked and the volumes used for extraction and clean-up in the procedure on the LOQ is the same as for the MDL.
- Stability of Reagents and Samples
- As stated earlier, all samples and analytical run standards must be analyzed within 48 hours of the digestion (24 hours recommended).
- All solutions for the analysis (other than the impinger solution) are stable for only two weeks because of probable contamination problems.
- Impinger solutions are stable for a maximum of one day because of precipitation of permanganate and the possibility of contamination.
16 References
- Varian Instruments at Work: Automated Cold Vapor Determination of Mercury: EPA Stannous Chloride Methodology, No. AA-51, September 1985.
- Van Delft, W. & Vos G., 1988. Comparison of Digestion Procedures for the Determination of Mercury in Soils by Cold-Vapour Atomic Absorption Spectrometry, Analytica Chimica Acta 209, 1988, p.147-156.
- Determination of ultratrace-level mercury in sediment and tissue by microwave digestion and atomic fluorescence detection. CEM reference R105.
- Comparison of a Microwave Digestion System to Other Digestion Methods for Plant Tissue Analysis. CEM reference RO 26.
- The Determination of Total Mercury (Hg) in Air Sampling Solutions, Regulation respecting Mercury (made under the Occupational Health and Safety Act, O. Reg. 23/87, 1987, p. 47-55.
17 Appendix
Appendix : Microwave Digestion
Parameters
Manufacturer: CEM
Model: MDS 2100 Digestion Vessel
Type: ACV - Advanced Composite Vessel
The following table includes a summary of the microwave digestion parameters. These include the power, pressure, temperature, time of the program and fan speed for the digestion of mainstream smoke samples in 5 stages.
| Stage: | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Power %: | 70 | 70 | 70 | 0 | 0 |
| Pressure (psi): | 50 | 125 | 175 | 20 | 150 |
| Run Time (min): | 20 | 15 | 20 | 20 | 20 |
| Time at Parameter: | 8 | 8 | 15 | 20 | 10 |
| Temperature: | 95 | 125 | 165 | 20 | 190 |
| Fan Speed: | 50 | 0 | 50 | 80 |
Note: The temperature and pressure parameters are set as the controlling parameters in this digestion program one of which will define the maximum reached. If either preset is not reached, the microwave oven delivers the designated power for the time programmed in the Run Time function.
Note: These are only suggested parameters as a starting point. The digestion procedure must be optimized for the specific application and instrument used.