Determination of "Tar" and Nicotine in Sidestream Tobacco Smoke
Health Canada
T-212, December 31, 1999
Table of Contents
- Scope of Applications
- Normative references
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- Modifications for Intensive Smoking Conditions
- References
1 Scope of Applications
- Applicable to the collection and quantitation of the tar and nicotine content of sidestream tobacco smoke.
2 Normative References
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Smoke, 1999-12-31.
- American Society for Testing and Materials (ASTM) D1193-77 - Standard Specifications for Reagent Water, Version 1977.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- This method describes the routine analysis of sidestream (SS) tobacco smoke using a British American Tobacco (BAT) fishtail chamber configuration. Sidestream smoke is all the smoke emitted from the lit end of a burning cigarette during the smoulder process. A glass fishtail chamber sits over a burning cigarette and allows the smoke to be directed in a controlled manner for the determination of sidestream particulate matter, water, nicotine and tar.
- Four conditioned cigarettes* are smoked per port, using a constant volume smoking machine. The SS smoke is collected on a glass fibre filter disc (pad). Sidestream total particulate matter (TPM), water, nicotine and tar are determined as specified in T-115.
*For other tobacco products, select a number such that breakthrough does not occur.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- Sidestream pad holders.
- Analytical balance measuring to at least four decimal places.
- Anti-static wipes.
- Tweezers and gloves for transferring pads.
- 50 mL amber serum bottles with stoppers.
- Desiccator.
- Constant rate platform shaker.
- Volumetric Flasks - 10, 25 and 50 mL.
- Volumetric pipettes or gas-tight syringe for range 100 to 1000 μL.
- Hewlett-Packard (HP) 5890 GC with FID and TCD and 6890 autosampler (or equivalent).
- Data collection system.
- Water Column - 6' X 1/8" o.d. (two metres X 3.2 mm o.d.) stainless steel - Poropak Type Q: 80 - 100 mesh.
- Nicotine Column - 6' X 1/8" o.d. (two metres X 3.2 mm o.d.) stainless steel - 16 % Apiezon L, 2 % KOH, 2 % Carbowax 20M on Chromosorb W: 80-100 mesh.
- UV Spectrophotometer - Spectronic Genesys 5 (or equivalent) with quartz cuvettes (1 cm path length).
- Vacuum pumps (GAST or equivalent).
- Flow Meter (15 mL capacity).
- Retort stand and clamps (one set per "fishtail").
- Impingers - 70 mL without frits.
- Tygon tubing.
- Electric lighter.
- Rubber bulb.
- Fishtail Chamber.
- Scintillation vials (10 mL) with foil-lined plastic caps.
- Screw-cap culture tubes (15 mL) with plastic caps.
- Glass funnels - 75 mm i.d., short stem.
- Rinsing rack.
- Pipette (200 μL-1000 μL), Pipette (1-5 mL).
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade in quality.
- Isopropanol.
- Methanol.
- Anethole (at least 99 % purity).
- Nicotine (at least 98 % purity).
- Type I Water - as specified in ASTM D1193.
- Glass fibre filter discs, 44 mm in diameter, with no more than 5 % acrylic type binder.
- Amber Autosampler vials with rubber septa lined caps.
- Disposable syringes - 5 mL.
- Syringe filters 0.45 μm, 25 mm diameter
- Parafilm® or equivalent.
- Disposable pipette tips.
- Wash bottles.
- Argon gas.
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
8 Preparation of Solutions
- Preparation of Extraction Solution
- Prepare extraction solution as specified in T-115.
9 Preparation of Standards
- Prepare standards as specified in T-115.
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars shall be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 (with the following modifications as detailed below).
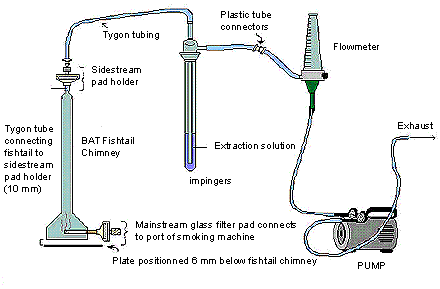
- Set up the Sidestream Apparatus as shown.
The following figure demonstrates sidestream apparatus. The BAT fishtail chimney contains a mainstream pad which connects to the port of the smoking machine at its base; whereas the tygon tube is connecting the fishtail to the sidestream pad holder on the top of the chimney. The sidestream pad holder on the BAT fishtail chimney is connected to the 250 mL impinger containing the extraction solution, by tygon tubing. The impinger is then connected outwards by plastic tube connectors to a flow meter, and is also further connected to a pump that controls the flow rate of the exhaust.
- Accurately transfer 30 mL of extraction solution into each impinger.
- Attach the SS holders to the top of the fishtail.
- Calibrate the flow meter to 3 L/minute.
- Insert cigarette into MS holder.
Note: Before lighting the cigarette, lower the fishtail to the smoking position. Adjust the alignment of the fishtail and cigarette so that they are not touching. Raise the fishtail and prepare to start run. - Start the run initiating the puff interval.
- At 30 seconds, turn on vacuum pump.
- At 51 seconds, light the cigarette using the lighter. Remove lighter immediately after puff has been taken.
- Position the bottom plate beneath the cigarette.
- Lower the fishtail over the cigarette to approximately 6 mm from the plate.
- Smoke the cigarette to the butt mark.
- Raise the fishtail and extinguish the cigarette using tweezers.
- Allow the pump to run for 30 seconds after the cigarette is extinguished to ensure all SS smoke is collected.
- Remove the cigarette butt.
- Repeat the smoking procedure for the remaining cigarettes.
Note: The number of cigarettes smoked for this analysis is four. Because of the large amount of TPM in SS smoke, only the smoke of the first two cigarettes is collected on the SS pad. After the second cigarette, the SS pad holder is removed, weighed for TPM and the pad is transferred to a 50 mL serum bottle. Another pad is placed into the SS pad holder and the run is continued. The second pad is disposed of at the end of the run. If the SS TPM levels are known to be very low, all four cigarettes may be smoked onto one pad.
13 Sample Generation
- Extraction of pads (SS)
- After two cigarettes are smoked, remove the SS pad holder from the smoking machine and weigh to determine TPM.
- Open the filter holder and, with gloves on and using clean tweezers, fold the pad into quarters, TPM inside.
- Wipe the internal surface of the holder with the clean surface of the pad and transfer the pad into a desiccated, labelled 50 mL amber serum bottle, TPM side up.
- Three blanks must be prepared with each smoking run. Place one conditioned pad into each of three desiccated 50 mL serum bottles and treat as samples.
- Add 20 mL of the extraction solvent to each serum bottle and seal with a stopper.
- Shake the bottles for 45 minutes on a platform shaker.
- Rinse two autosampler vials with the contents of each bottle, then fill each vial, cap and label with run #, port #, and A or B and place A samples on GC autosampler tray for analysis.
- Store B samples in the dark to be used if necessary.
- Filter a portion of the SS pad extract into a vial using a syringe and filter. Store until ready to do Tar analysis on the Spectrophotometer.
- Rinsing of Fishtail Chamber
- At the end of the run, remove the fishtail from the clamps and place upside down into a rack for rinsing.
- Place a 50 mL volumetric flask beneath the fishtail.
- Place a glass funnel into the 50 mL volumetric.
- Rinse the fishtail down into the volumetric flask using a squirt bottle containing extraction solution.
- Make the flask to volume with extraction solution, stopper and mix well.
- Decant the solution into two GC vials (A and B) for nicotine analysis.
- Pour the remaining solution into scintillation vials. Store until ready to do Tar analysis on the Spectrophotometer.
- Impinger Solution
- Rinse the impinger solution through the Tygon tubing and pour into a 50 mL volumetric flask.
- Rinse the impinger and tubing twice with 5 mL aliquots of extraction solution and transfer to the flask.
- Make the flask to volume with extraction solution, stopper and mix well.
- Decant the solution into two GC vials (A and B) for nicotine analysis.
14 Sample Analysis
- GC Analysis
- Typical GC Conditions:
Oven Temperature: 190 °C.
Injector Temperature: 230 °C.
Detector Temperature: 230 °C.
Carrier Gas: Purified Helium @ pressure 60 psi.
Flow Rates
FID: Column flow: 20 mL/minute.
Column + Hydrogen: 60 mL/minute.
Column + Air + Hydrogen: 350 mL/minute.
TCD: Column flow: 20 mL/minute.
Column + Reference: 30 mL/minute. - Two μL of each standard and sample are injected onto the GC for nicotine analysis. Only the sidestream pad extracts are analyzed for water.
- Typical GC Conditions:
- Spectrophotometric Analysis
- Turn on the spectrophotometer and set the wavelength to 310 nm.
- Zero the spectrophotometer with a "blank" cuvette containing extraction solution.
- Measure and record the absorbance of the SS pad extract and the Fishtail rinse. Make dilutions as necessary with extraction solution so that the absorbance of all solutions falls between 0.2 and 0.8. Record any dilutions to be used in the final calculation.
Note: Typical dilutions are 0.25-0.50 mL to 10 mL for the SS pad extract and 2.5-5.0 mL to 10 mL for the fishtail solution.
- Calculations
- Calibration Curve
- With each new batch of extraction solution prepared, the GC must be recalibrated to determine new slopes and intercepts for water and nicotine calculations as well as to monitor any changes in GC performance. Each recalibration involves preparing new stock and standard solutions.
- Sample Calculations
- TPM (Sidestream Pad)
TPM (mg/cig) = ([CFH after(g) - CFH before(g)] × 1000(mg/g)) / 2 (cigarettes).
Note: the number of cigarettes may be four for very low SS TPM brands. - Water (SS Pads)
Water results are calculated from the calibration curve and are reported in mg/cigarette. The water content of the three blanks is determined from the calibration curve for water. The average of the three blanks is subtracted from the water results for that run. - Nicotine (SS Pads, Fishtail and Impinger Solutions)
Nicotine results are calculated from the calibration curve and are reported in mg/cigarette. - Tar (SS Pads)
The Tar value is determined for each observation by the following:
Tar (mg/cigarette) = TPM - Nicotine - Water. - Tar (Fishtail)
The Fishtail Tar value is calculated from the following equation:
FT Tar (mg/cigarette) = (SS Tar × FT Abs × FT DF)/(SS Abs × SS DF).
where: SS Tar is the tar value obtained from the SS pad.
FT Abs is the absorbance of the fishtail solution.
FT DF is the dilution factor of the fishtail solution.
SS Abs is the absorbance of the SS pad extract.
SS DF is the dilution factor of the SS pad extract.
- SS Tar and Nicotine
The total SS tar and nicotine are calculated as follows:
SS Tar (mg/cigarette) = SS Pad Tar + FT Tar.
SS Nicotine (mg/cigarette) = SS Pad Nicotine + Fishtail Nicotine + Impinger Nicotine.
- TPM (Sidestream Pad)
15 Quality Control
- Recoveries and Levels of Contamination
- Laboratory Reagent Blanks (LRB) are used to monitor the level of water and nicotine contamination in the reagents (including glassware and pads). Although nicotine is typically ND in these blanks, there is always some water due to the presence of the water in the extraction solution and the conditioned pad.
- Laboratory Fortified Blanks (LFB) are used to evaluate the extent of potential analyte loss during the extraction process. LFBs should be run whenever there is a question about the validity of results.
- Method detection limit (MDL)/Limit of Quantitation (LOQ)
This involves the use of either a test material with a low level of the analyte or the lowest standard. The standard deviation is then determined and the MDL is determined to be three times the standard deviation. LOQ is taken as 10 times the standard deviation. - Stability of Reagents and Samples
- Extraction solution is stable but can become contaminated with water over time. For this reason, and to ensure nicotine calibration remains constant, fresh standards for nicotine and water should be made weekly.
16 Modifications for Intense Smoking Conditions
- Under intense smoking conditions, the number of cigarettes smoked per port is two.
17 References
- Proctor, C.J., Martin, C., Beven, J.L., and Dymond H.F., 1988. Evaluation of an Apparatus Designed for the Collection of Sidestream Tobacco Smoke, Analyst 113: p. 1509-1513.
- Cigarettes - Sampling, International Reference Number ISO 8243:1991.
- Tobacco and tobacco products - Atmosphere for conditioning and testing, International Standard Reference Number ISO 3402:1991 (E).
- Routine analytical cigarette-smoking machine - Definitions and standard conditions, International Standard Reference Number ISO 3308:1991 (E).
- Cigarettes - Determination of total and nicotine-free dry particulate matter using a routine analytical cigarette smoking machine, International Standard Reference Number ISO 4387:1991 (E).
- Cigarettes - Determination of nicotine in smoke condensates - Gas-chromatographic method, International Standard Reference Number ISO 10315: 1991 (E).
- Cigarettes - Determination of water in smoke condensates - Part 1: Gas-chromatographic method, International Standard Reference Number ISO 10362-1:1991 (E).
- Cigarettes - Determination of carbon monoxide in the vapour phase of cigarette smoke - NDIR method, International Standard Reference Number ISO 8454:1995 (E).