Determination of Nitrosamines in Sidestream Tobacco Smoke
Health Canada
T–209, December 31, 1999
Table of Contents
- Scope of Applications
- Normative References
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- Modifications for Intensive Smoking
- References
- Appendices
1 Scope of Applications
- The volatile nitrosamines (VNA) are formed during the processing of tobacco and during the smoking of tobacco products. The tobacco processing methods include air-, sun-, flue- and fire-curing, ageing and fermentation.
- The generation of sidestream smoke is achieved under standard machine smoking conditions for cigarettes as specified in T-115. Sidestream smoke is effectively, all smoke emitted from the cigarette, other than mainstream smoke.
- This method is suitable for the quantitative determination of four tobacco specific N-nitrosamines (TSNA) in sidestream tobacco smoke (SS): N-nitrosonornicotine (NNN), 4-(N-nitrosomethylamino)- I -(3-pyridyl)- 1 -butanone (NNK), N-nitrosoanatabine (NAT) and N-nitrosoanabasine (NAB).
2 Normative References
- American Society for Testing and Materials (ASTM) D1193-77 - Standard Specifications for Reagent Water, Version 1977.
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- Four* cigarettes are smoked individually beneath a British American Tobacco (BAT) fishtail chamber coated with ascorbic acid at a 2 L/minute flow rate in order to capture the sidestream (SS) smoke.
- The SS smoke passes through an aqueous buffer, containing ascorbic acid, which prevents formation of TSNA by scavenging oxides of nitrogen. The SS smoke is then trapped onto a pre-treated (with ascorbic acid) 44 mm glass fibre filter disc (pad).
- The TSNA are concentrated by combining the dichloromethane extraction of the aqueous buffer and Cambridge filter pad, followed by column chromatography onto basic alumina.
- The fraction containing TSNA is eluted, then quantitatively analyzed by combined gas chromatography-thermal energy analysis (GC-TEA). N-nitrosoguvacoline (NG) is used as an internal standard.
*For other tobacco products, select a number such that breakthrough does not occur.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
All four TSNA are carcinogenic in several species of laboratory animals. Extreme care should be taken in handling these compounds. The exhaust of the TEA detector should be vented properly in order to reduce exposure to possible excess ozone (03).
5 Apparatus and Equipment
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- 250 mL Round-bottom flask with ground glass joint.
- Volumetric Flask 2, 10, 50, 100, 200 mL.
- 100 mL Beaker.
- Wrist action shaker.
- LC Column with Frit and Stopcock, 300 mm X 22 mm ID X 25 mm OD (Supelco 64754) or equivalent.
- Silinized Glass Wool.
- Short Stem Glass Funnels.
- 250 mL Separatory Funnels.
- Glass Pasteur Pipettes.
- Zymark TurboVap II Concentrator equipped with 200 mL tubes with graduated 1 mL stem or equivalent.
- Thermal Energy Analyzer (Thermo-Electron Corp.) interfaced to GC or equivalent.
- GAST Pumps or equivalent.
- Ace flow meters or equivalent.
- BAT Fishtail Chambers.
- 70 mL impingers with ground glass joints and Teflon sleeves.
- Gas chromatograph, equipped with temperature programmable injector, electronic flow control and data processing software.
- GC column, 30 m X 0.32 mm X 3.0 μm DB-1 fused silica capillary column.
- Non-ultra violet (UV) lighting.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade in quality.
- Dichloromethane - Distilled in Glass (DIG).
- Acetone - DIG.
- Sodium sulfate - Anhydrous.
- Basic Alumina.
- Citric Acid (Anhydrous).
- L-Ascorbic acid.
- Sodium Phosphate Dibasic.
- Methano - DIG.
- Type I water.
- Aluminum foil.
- Petri dish.
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
8 Preparation of Solutions
- 1:1 Acetone:Dichloromethane solution
- Mix Acetone and Dichloromethane in a 1:1 v/v basis.
- Citrate-Phosphate Buffer containing L-Ascorbic Acid
- Prepare a 1 L solution with Type I water containing :
- 55 mM Citric Acid.
- 90 mM Sodium Phosphate Dibasic.
- 20 mM L-Ascorbic Acid.
Note: pH of the solution must be between 4.3 and 4.5 or it must be remade.
- Prepare a 1 L solution with Type I water containing :
- Aqueous L-Ascorbic Acid Solution for SS Pad Pre-treatment
- Weigh 33.33 g of L-ascorbic Acid into a 200 mL volumetric flask.
- Make up to volume with Type I water.
Note: If the solution has yellowed, the solution should be re-made.
9 Preparation of Standards
- N-nitrosoguvacoline (NG) internal standard
- Prepare a solution at 5000 ng/mL in dichloromethane.
- TSNA mixed standard solution
- Prepare a mixed standard dilution stock solution of NNN, NAT, NAB and NNK in dichloromethane at the following range of concentrations:
- NNK at 3000 ng/mL.
- NNN and NAT at 1500 ng/mL.
- NAB at 500 ng/mL.
Note: Concentrated solutions are stable for approximately six months if stored at -20 °C in such a manner as to prevent loss of solvent from evaporation.
- Build a calibration ranging from approximately 20 ng/mL (for NAB) to 2000 ng/mL (for NNK) containing NG as an internal standard at 500 ng/mL in each of the standards.
- Representative calibrations are in Appendix V.
Note: Individual calibration stocks are stable for two months if properly stored in a freezer.
- Prepare a mixed standard dilution stock solution of NNN, NAT, NAB and NNK in dichloromethane at the following range of concentrations:
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product is to be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars are to marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Non-UV lighting shall be used in the rooms in which sample generation and sample analyses are conducted.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 with the following modifications as detailed below:
- Pre-treatment of SS Collection Pad.
- Saturate the SS collection pad with the aqueous L-ascorbic acid solution by placing the pad in a Petri dish with the solution for 15 seconds, turning over and allowing to sit for an additional 15 seconds.
- Place the pads in a rack in the CER for conditioning for 24-48 hours to allow the pads to dry and equilibrate.
Note: A slight yellowing of the pad will occur over the 48 hours and the pad will feel brittle.
- Pre-treatment of BAT Fishtail
- Prepare an L-Ascorbic acid slurry with methanol in a 100 mL beaker.
- Pour the slurry down the fishtail from the cigarette end to the top of the fishtail to create an even coat over the entire inner surface of the fishtail.
Note: The thickness of the coat should be such that one cannot look through the fishtail.
- SS Smoke Generation and Trapping
- Prepare two 70 mL impingers each containing 25 mL of the citrate-phosphate buffer containing L-ascorbic acid.
- Connect these two impingers in series to the exit of the BAT fishtail and place a treated 44 mm Cambridge filter pad after the second impinger, as per the illustration.
- Place a regulating flow meter between the Cambridge filter and a constant vacuum source and adjust the flow rate to 2 L/minute.
Sidestream Apparatus Set-Up Diagram
The following figure demonstrates sidestream apparatus. The BAT fishtail chimney contains a mainstream glass filter pad which connects to the port of the smoking machine at its base. Inside, the BAT Fishtail chimney is treated with L-ascorbic acid. The sidestream pad holder on the BAT fishtail chimney is connected by tygon tubing to two 70 mL impingers in series, each containing citrate phosphate buffer. The second impinger is then connected outwards to a device that treats the sidestream tobacco smoke filter pad for collection purposes, and is also further connected to a rotameter followed by a pump that controls the flow rate of the exhaust.
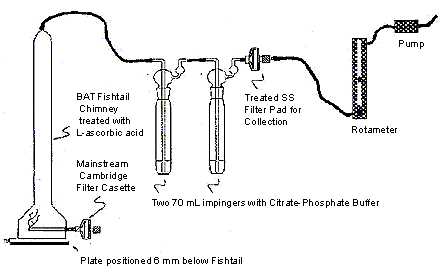
13 Sample Generation
- Place a cigarette in the cigarette holder connected to the piston type smoking machine.
- Light the cigarette and lower the sidestream apparatus over the cigarette placing a metal plate 6 mm below the edge of the fishtail. Smoke the cigarette as per T-115.
- Smoke four cigarettes in this manner, capturing the sidestream smoke, to make one sample.
14 Sample Analysis
- TSNA Extraction and Concentration
- Immediately after smoking, place the SS filter pad into a 250 mL round bottom flask wrapped with aluminum foil.
- Add 100 μL of internal standard solution to the flask.
- Combine the buffer solutions from the two impingers into a 250 mL separatory funnel.
- Wash (rinse) each of the impingers with 25 mL (total of 50 mL) of dichloromethane and add to the separatory funnel.
- Shake the separatory funnel to extract the TSNA.
- Drain the dichloromethane layer into the round bottom flask containing the SS filter pad.
- Repeat the extraction (12.1.5 to 12.1.6) with two additional 50 mL portions of dichloromethane (total extraction volume 150 mL).
- Extract the pad by placing the flask on a wrist action shake for 30 minutes until the SS pad is completely broken up.
- Place a plug of silanized glass wool into the stem of a glass funnel.
- Add approximately 2 g of anhydrous sodium sulphate (1 cm depth in funnel).
- Pour the extract from the 250 mL round bottom flask through the funnel directly into a 200 mL TurboVap tube.
- Rinse the flask with 2 X 20 mL portions of dichloromethane, passing the rinses through the funnel to aid in transfer of all of the extracted TSNA to the TurboVap tube.
- After all solution has stopped dripping through the funnel, place the sample into the TurboVap II Concentrator set at 38 °C and 10 psi nitrogen.
- Concentrate samples to approximately 5 mL.
- Column Chromatography Clean-up Procedure
- Prepare Basic Alumina Column by adding 50 mL of DCM to an empty, dry, glass LC column.
- Add 10 g (+/- 0.2) of oven dried (110 °C) basic alumina to the liquid in the column. Stir the alumina slurry with a glass rod to remove any possible air pockets.
- Drain the liquid from the column to waste, closing the stopcock when the solution is at the level of the alumina.
- Wash the alumina by adding 50 mL of DCM to the column. Drain the liquid to waste and close the stopcock when the solution is at the level of the alumina.
- Add the 5 mL sample from the TurboVap tube to the alumina column using a glass Pasteur pipette attempting not to disturb the alumina packing.
- Drain the liquid from the column to waste, closing the stopcock when the solution is at the level of the alumina.
- Rinse the TurboVap tube with 10 mL DCM, washing the lower portion (25 %) of the tube with repeated flushing using a Pasteur pipette.
- Add the 10 mL rinse from the TurboVap tube to the alumina column using a glass Pasteur pipette attempting not to disturb the alumina packing.
- Drain the liquid from the column to waste, closing the stopcock when the solution is at the level of the alumina.
- Rinse the TurboVap tube and alumina column with an additional 40 mL DCM attempting not to disturb the alumina packing, draining the liquid from the column to waste.
- Place a clean 200 mL TurboVap tube beneath the LC Column to collect the sample.
- Elute the TSNA from the alumina column by adding 50 mL of 1:1 Acetone:DCM to the column attempting not to disturb the alumina packing.
- Collect the liquid from the column into the TurboVap tube, closing the stopcock when the solution is at the level of the alumina.
- Repeat steps 14.2.12 and 14.2.13 four more times, collecting the eluent in the same TurboVap tube (a total of 250 mL collected).
Note: This will require evaporating a portion of the eluent before the final 50 mL can be collected into the tube.
- Sample Re-Concentration
- Place the samples into the TurboVap II Concentrator set at 38 °C and 9 psi nitrogen.
- After the samples have been concentrated to approximately 150 mL, increase the pressure to 10 psi.
- Concentrate samples to 0.8 mL or when the sensor turns the concentration off (approximately 45 minutes).
- Add dichloromethane to the tube to the 1.0 mL graduation of the tube.
- Rinse the lower portion (25 %) of the tube with the final extract to dissolve any residue that may have dried to the side of the tube.
- Transfer the sample to an amber autosampler vial with Teflon lined septa for GC analysis.
- GC-TEA Analysis
- GC-TEA Operating Conditions
- Carrier flow rate (He): 2.8 mL/minute using electronic flow control (velocity = 60 cm/second).
- Injector temperature: Programmable 35 to 220 °C.
- Oven temperature: Programmed 50 to 170 to 212 °C.
- TEA interface temperature: 240 °C.
- TEA furnace temperature: 500-525 °C (dependent on analyzer sensitivity).
- Analysis Run Time: 35 minutes.
- Blank test
- Blank tests using purified nitrosamine-free air should be performed periodically in order to ensure the absence of nitrosamine traces in the analytical environment, or their formation during analysis.
- GC-TEA Calibration
- Inject 1.5 µL of the TSNA mixed standard solution and determine peak areas for the four components.
- TSNA determination
- Inject 1.5 µL of the sample concentrate (14.3.6) and determine areas of the peaks having retention times corresponding to NNN, NAT, NAB and NNK.
- GC-TEA Operating Conditions
- Method of Calculation
- The content, mcig (ng/cigarette), of a given TSNA is obtained from:
mcig = CVs/N
where
C = analytical Concentration determined by ISTD calibration of given TSNA.
Vs = final volume of concentrate.
N = number of cigarettes/little cigars/cigars smoked.
- The content, mcig (ng/cigarette), of a given TSNA is obtained from:
15 Quality Control
- Typical Chromatogram
- See Appendix 1.
- Method Detection Limits (MDL) / Limit of Quantitation (LOQ)
- The method detection limit (MDL) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The MDL is calculated as three times the standard deviation of these determinations.
- The MDL (on a ng/cigarette basis) can be calculated by multiplying the determined MDL (ng/mL basis) by the final volume of the extract and dividing this by the number of cigarettes smoked.
- The MDL (on a ng/cigarette basis) can be enhanced by modifying the number of cigarettes smoked and the volumes used for extraction and clean-up in the procedure.
- The practical limit of quantitation (LOQ) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The LOQ is calculated as 10 times the standard deviation of these determinations.
- The LOQ (on a ng/cigarette basis) can be calculated by multiplying the determined LOQ (ng/mL basis) by the final volume of the extract and dividing this by the number of cigarettes smoked.
- The effect of varying the number of cigarettes smoked and the volumes used for extraction and clean-up in the procedure on the LOQ is the same as for the MDL.
16 Modifications for Intensive Smoking
- None required.
17 References
- Adams, J.D., Brunnemann, K.D. & Hoffmann, D., 1983. Chemical studies on tobacco smoke. LXXV. Rapid method for the analysis of tobacco-specific N-nitrosamines by gas-liquid chromatography with a thermal energy analyser. J. Chromatogr., p. 256, 347-351.
- Brunnemann, K.D. & Hoffmann, D., 1981. Assessment of the carcinogenic N-nitrosodiethanolamine in tobacco products and tobacco smoke. Carcinogenesis, p. 2, 1123-1127.
- Hecht, S.S., Adams, J.D. & Hoffmann, D., 1983. Tobacco-specific nitrosamines in tobacco and tobacco smoke. In: Preussmann, R., O'Neill, I.K., Eisenbrand, G., Spiegelhalder, B. & Bartsch, H., eds, Environmental Carcinogens- Selected Methods of Analysis, Vol. 6, N-Nitroso Compounds (IARC Scientific Publications No. 45), Lyon, International Agency for Research on Cancer, p. 93-101.
- Hoffmann, D., Adams, J.D., Brunnemann, K. D. & Hecht, S. S., 1979. Assessment of tobacco-specific N-nitrosamines in tobacco products. Cancer Res., p. 39, 2505-2509.
- Risner, Charles H., and Wendelboe, Fred N., 1994. Quantification of Tobacco Specific Nitrosamines in Tobacco, Tob. Sci. 38: p.1-6.
18 Appendices
Appendix 1: Typical Chromatogram
The following figures are typical chromatogram of sidestream tobacco smoke extract for 1R4F, where the mVolts of the apparatus are plotted against the time (minutes).
A typical chromatogram of the SS extract for 1R4F.
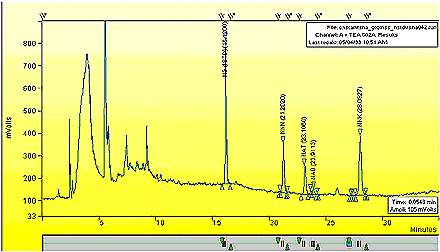
See the next image for an expanded region of the above chromatogram.
