Determination of 1,3-Butadiene, Isoprene, Acrylonitrile, Benzene, Toluene and Styrene in Sidestream Tobacco Smoke
Health Canada
T-213, December 31, 1999
Table of Contents
- Scope of Applications
- Normative references
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Calculations
- Quality Control
- Reference
1 Scope of Applications
- Applicable to the isolation and quantitation of the 1,3-Butadiene, Isoprene, Acrylonitrile, Benzene, Toluene and Styrene ("Volatiles") content of sidestream tobacco smoke.
2 Normative References
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide (CO) in Mainstream Tobacco Smoke, 1999-12-31.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- This method is used for the analysis of sidestream (SS) tobacco smoke using a fishtail chimney configuration. Sidestream smoke is all the smoke emitted from the lit end of a burning cigarette during the smolder process. The glass fishtail chimney sits over a burning cigarette and allows the smoke to be directed in a controlled manner for the determination of sidestream tobacco constituents.
- The sidestream smoke of two cigarettes* is drawn through a fishtail chamber and passed through a glass fibre filter pad which is subsequently used for sidestream (SS) Total Particulate Matter (TPM) determination. The gas phase is led through three 70 mL impingers, each containing methanol. The first trap remains at room temperature while the second and third traps are kept at or below -70 °C in a dry ice/isopropanol bath. The impinger solutions are pooled, spiked with D6-benzene and injected onto a GC/MS for quantitation.
*For other tobacco products, select a number such that breakthrough does not occur.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T-115.
- Analytical balance measuring to at least four decimal places.
- Vacuum pumps (GAST or equivalent).
- Victor Flow meter or equivalent.
- BAT (British American Tobacco) Fishtail Chimney mounted on a retort stand.
- Tygon tubing.
- 70 mL glass impingers with extra-coarse frits.
- 10, 25, 50 and 100 mL volumetric flasks.
- Volumetric pipettes or gas-tight syringes for range 100 to 1000 μL.
- Screw-cap autosampler vials with caps and Teflon-lined septa.
- Varian Saturn I GC/MS system consisting of an 8100 autosampler, a 3400 GC with a 1077 split/splitless injector and an ion trap detector (or equivalent).
- J&W Scientific 60 m X 0.32 mm X 1 μm DB-5MS column (or equivalent) with 1 m X 0.25 mm deactivated fused silica transfer line.
- Dewar flasks.
- Pasteur pipettes.
- Vortex.
- Thermometer (-100 to 40 °C).
- Spectrophotometer.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade quality.
- Dry ice.
- Propan-2-ol (IPA).
- Methanol (Distilled-in-Glass).
- Reagant Alcohol (Distilled-in-Glass).
- D6 - Benzene - Purity of D6 > 99 %.
- 1,3-butadiene.
- Isoprene.
- Acrylonitrile.
- Benzene.
- Toluene.
- Styrene.
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
8 Preparation of Standards
- Preparation of Standards (except 1,3-butadiene)
- Five primary stock solutions are prepared by accurately weighing 100 μL each of isoprene, acrylonitrile, benzene, toluene and styrene into five 10 mL volumetric flasks, filling each flask to the mark with methanol and mixing well.
- A combined secondary stock solution is prepared by transferring appropriate aliquots of each of the primary stock solutions into a 25 mL volumetric flask, filling it to the mark with methanol and mixing well.
- A stock solution of D6-benzene is prepared by transferring the contents of a 1 g ampoule to a 10 mL volumetric flask, filling the flask to the mark with methanol and mixing well.
- An internal standard spiking solution is prepared by diluting 4 mL of the stock to 100 mL with methanol and mixing well. Aliquots of this spiking solution are stored in 25 mL vials with Teflon-lined caps and at minus 20 °C.
- Five calibration standard solutions are prepared by adding 100 μL ISTD to each of five 10 mL volumetric flasks. The sides are rinsed with methanol, then appropriate aliquots of the secondary stock solution are added to each flask. The flasks are filled to the mark with methanol and mixed well.
- The solutions are transferred to a series of labeled autosampler vials, capped with Teflon-lined septa and stored at minus 20 °C until use.
Note: Each vial is only used once.
- Preparation of 1,3-Butadiene Standards
- Attach a piece of Tygon tubing to the valve of a 1,3-butadiene cylinder. Place a Pasteur pipette on the other end and immerse the tip of the pipette into a 100 mL volumetric flask containing methanol up to the base of the neck of the flask. Open the valve and gently bubble the 1,3-butadiene into the methanol for about five minutes. Make the volume to the mark with methanol and mix well.
- Pipette 1 mL of the stock solution into a clean 100 mL volumetric flask and make to the mark with methanol and mix well. This is the secondary stock solution.
- Determination of Secondary Stock Concentration
- Pipette 1 mL of the secondary stock solution into a 100 mL volumetric flask and make to the mark with reagent alcohol and mix well.
- Measure the absorbance of the solution against a reagent alcohol blank on the spectrophotometer at 217 nm. Make dilutions as necessary so that the absorbance (A) falls between 0.2 and 0.6.
- Calculate the concentration of the secondary stock solution according to the following:
Conc. (µg/mL) = [A / (20 893 L/mole)] × (54 g/mole) × [(1000 mg/g) / (1000 mL/L)] × [(100 mL) / (1 mL)] × (1000 µg/mg)
- Once the concentration of the secondary stock solution is known, make a minimum of four calibration standard solutions in the range appropriate for the expected delivery levels (typically five to 50 µg/mL).
- Add internal standard solution (100 μL) to each 10 mL volumetric. Add an appropriate aliquot of secondary stock solution, and make up to the mark with methanol.
- Transfer the solutions to a series of labeled autosampler vials. Cap with Teflon-lined septa and store at minus 20 °C until use.
Note: Each vial is used only once.
9 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
10 Tobaco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars shall be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
11 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 (with the following modifications as detailed below:)
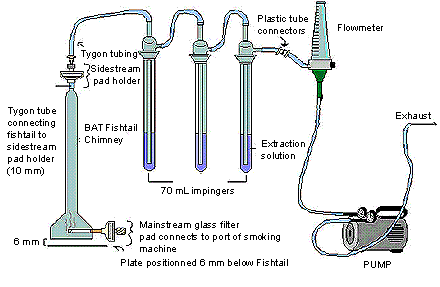
The following figure demonstrates sidestream apparatus. The BAT fishtail chimney contains a mainstream pad which connects to the port of the smoking machine at its base; whereas the tygon tube is connecting the fishtail to the sidestream pad holder on the top of the chimney. The 10 mm sidestream pad holder on the BAT fishtail chimney is connected to three 70 mL impingers in series containing the extraction solution, by tygon tubing. The last impinger is then connected outwards by plastic tube connectors to a flow meter, and is also further connected to a pump that controls the flow rate of the exhaust.
- The machine conditions shall be as those specified in T-115 (with the following modifications as detailed below:)
12 Sample Generation
- Smoking is conducted using between four and eight ports of a linear 20 port smoking machine. The sidestream smoke is collected by a fishtail chamber mounted above the cigarette.
- Insert the mainstream (MS) pad holder with pad into the assigned port of the smoking machine.
- Prepare the impingers by adding 10 mL of methanol to the first impinger and 20 mL of methanol into the second and third impingers.
- Immerse the second and third impingers into a dry-ice/IPA bath (temperature at or below -70 °C) leaving the first at room temperature.
Note: The first impinger is left at room temperature due to the large amount of water in the sidestream smoke which causes the impingers to freeze in the dry ice/IPA bath. - Position the SS filter pad holder above the fish-tail chamber and then hook up in series three impingers to the filter pad holder. Connect the tubing of the last impinger to the flow meter. Connect the flow meter to the vacuum pump.
- Turn the pump on (flow rate of 2 L/minute) just prior to lighting the cigarette. Light the cigarette and initiate the puff count.
- Lower the chimney to its lowest position. Do not allow the cigarette to touch the chimney. Keep the chimney approximately 6 mm from the plate insert.
- Burn the cigarette to the previously marked standard butt length. Remove the butt and save if required. After the cigarette has been smoked to the line, leave the pump on for approximately 20 seconds to collect all of the smoke from the fishtail chimney. Raise the chimney to its highest position and turn the pump off.
- Smoke the second cigarette in the same manner as the first.
- After smoking two cigarettes per port, remove both mainstream and sidestream filter holders and record their final weight on the run sheet to obtain the MS and SS TPM. Total particular matter (TPM) is determined as described in T-115. Data for mainstream and sidestream TPM is used to characterize samples and to monitor the smoking process.
13 Sample Anylasis
- Immediately after smoking is completed, pool the contents of the three impingers and spike with 500 μL ISTD solution. Vortex for approximately 10 seconds.
- Decant aliquots of each impinger solution into two labeled autosampler vials. Fill each vial to the base of the neck and cap with an autosampler cap and Teflon-lined septum. DO NOT OVERFILL VIALS.
- Store samples at minus 20 °C for up to 48 hours prior to anal
- ysis.
- Instrument Analysis: GC/MS Conditions
Injector temperature: 20 °C.
Column temperature: 35 °C for five minutes.
20 °C/minute to 225 °C, hold six minutes.
Column pressure: 13 psi.
Transfer line temperature: 240 °C.
Manifold temperature: 240 °C. - One μL of the methanol solution is injected onto the GC/MS, which is run in the split mode (split flow 30 mL/minute).
- The GC/MS is operated in full-scan mode (50 to 200 amu). The following ion peak areas are used for quantitation:
1,3-butadiene 51+52
Isoprene 67
Acrylonitrile 52
Benzene 78
D6 Benzene 84
Toluene 91
Styrene 104
Note: The assignment of these masses is based on selection of the best response (i.e. the base peak) and the need to avoid possible contamination from interfering peaks which may contain similar ions. The choice of quantitation ions may be different for different instrument configurations.
14 Calculations
- Calibration Curve
- A calibration curve is generated at the beginning of each sample set or "project". Each standard solution is injected once and a calibration file built using the method for internal standard quantitation available with the Saturn quantitation software.
- A check standard is analyzed every 20 samples and at least once per run. This standard is treated as a sample and the observed value is compared to the expected value for that standard. A difference of more than 10 % of expected requires the following course of action.
- Make fresh calibration standards and run as check standards.
- If the results are within 10 % of expected, the first set of standards should be discarded and the new set used. The calibration is still valid.
- If the results differ by more than 10 % of expected, the calibration is no longer valid and a new calibration curve must be generated.
- Sample Calculation
- The software on the GC/MS is used to generate results for each analyte based on the concentrations of the standard solutions. The results are reported in μg/mL. To calculate the final results, the following calculation is used:
Analyte (µg/cigarette) = [Conc. of Analyte in Sample (µg/mL) × Volume (mL)] / No. of cigarettes
- The software on the GC/MS is used to generate results for each analyte based on the concentrations of the standard solutions. The results are reported in μg/mL. To calculate the final results, the following calculation is used:
15 Quality Control
- Recoveries and Levels of Contamination
- To determine trapping efficiency, the three impingers may be analyzed separately. The amount of each analyte is determined in each impinger and is reported as a % of the total.
- Laboratory reagent blanks (LRB) should be analyzed every 20 samples. There is occasionally a small amount of toluene present in methanol and this should be monitored closely. A laboratory fortified blank (LFB) is not necessary for this analysis as there is no sample work-up.
- In lieu of an LRB, a smoking blank can be used to monitor contamination of reagents and the air in the smoking room. This involves conducting a smoking run with the same number of puffs as a control cigarette but with no cigarette in place.
- Method Detection Limit (MDL)/Limit of Quantitation (LOQ)
- The MDL can be defined as the level that gives a signal to noise ratio of three to one. The LOQ can be defined as the level that gives a signal to noise ratio of 10 to one. Because of chromatographic differences and the effect of solvent on the 1,3-butadiene peak, each analyte has different MDL and LOQ. They are estimated as follows (units µg/mL):
Note: The DB-5 column used in this method would not be the best choice for analyzing acrylonitrile alone. It gives a poor peak shape and this is reflected in the much higher MDL and LOQ for this analyte. A more polar column would be more suitable for acrylonitrile, but for most purposes, the detection limits are acceptable for the matrices covered by this test method.MDL LOQ 1,3-butadiene 0.3 1 Isoprene 0.05 0.2 Acrylonitrile 0.3 1 Benzene 0.05 0.2 Toluene 0.05 0.2 Styrene 0.05 0.2
- The MDL can be defined as the level that gives a signal to noise ratio of three to one. The LOQ can be defined as the level that gives a signal to noise ratio of 10 to one. Because of chromatographic differences and the effect of solvent on the 1,3-butadiene peak, each analyte has different MDL and LOQ. They are estimated as follows (units µg/mL):
- Stability of Reagents and Samples
- Volatile mix standards are stable for at least one week if kept at minus 20 °C. Once punctured, the Isoprene is lost rapidly so each vial is used only once.
- 1,3-butadiene standards are stable for approximately one week if kept at minus 20 °C. Once punctured, the vial is discarded.
- Volatile stock solutions should be made fresh at the beginning of every project and can be stored in the freezer for at least two weeks to be used for working standards.
- Acrylonitrile, benzene, toluene and styrene are significantly less volatile than 1,3-butadiene and isoprene, and stock solutions may be stable for up to a month if kept at minus 20 °C.
- The secondary stock solution for 1,3-butadiene can be re-used almost indefinitely as the actual concentration of this solution is determined every time working standards are prepared from it.
- Samples are stable at minus 20 °C for up to 48 hours if the septum has not been punctured. I t is essential that at least two vials be prepared for each sample as the vial is discarded once punctured.
16 References
- Byrd, G.D., K.W. Fowler, R.D. Hicks, M.E. Lovette and M.F. Borgerding, 1990. Isotope dilution gas chromatography-mass spectrometry in the determination of benzene, toluene, styrene and acrylonitrile in mainstream cigarette smoke. J. Chromat. 503, p. 359-368.
- Brunnemann, K.D., M.R. Kagan, J.E. Cox, and D. Hoffmann, 1990. Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Carcinogenesis 11, p. 1863-1868.
- Brunnemann, K.D., M.R. Kagan, J.E. Cox, and D. Hoffmann, 1989. Determination of benzene, toluene and 1,3-butadiene in cigarette smoke by GCMSD. Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Exp. Pathol. 11, p. 108-113.