Determination of Nitrosamines in Whole Tobacco
Health Canada
T-309 December 31, 1999
Table of Contents
- Scope of Applications
- Normative References
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solution
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Sample Analysis
- Quality Control
- References
- Appendix
1 Scope of Applications
- The volatile nitrosamines (VNA) are formed during the processing of tobacco and during the smoking of tobacco products. The tobacco processing methods include air-, sun-, flue- and fire-curing, ageing and fermentation.
- This method is suitable for the quantitative determination of four tobacco specific N-nitrosamines (TSNA) in whole tobacco: N-nitrosonornicotine (NNN), 4-(N-nitrosomethylamino)- I -(3-pyridyl)- 1 -butanone (NNK), N-nitrosoanatabine (NAT) and N-nitrosoanabasine (NAB).
2 Normative References
- American Society for Testing and Materials (ASTM) D 1193-77 - Standard Specification for Reagent Water, Version 1977.
- Health Canada Test Method T-402 - Preparation of Cigarettes, Cigarette Tobacco, Cigars, Kreteks, Bidis, Package Leaf, Pipe and Smokeless Tobaccos for Testing, 1999-12-31.
- Health Canada Test Method T-115 - Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
3 Definitions
- Refer to T-115 for definitions of terms used in this document.
4 Method Summary
- TSNA are extracted using an aqueous buffer, containing ascorbic acid, which prevents artifact formation. The TSNA are enriched by extraction with dichloromethane, followed by column chromatography. The fraction containing TSNA is then quantitatively analysed by combined gas chromatography-thermal energy analysis (GC-TEA). N-nitrosoguvacoline (NG) is used as an internal standard.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
Note: All four TSNA are carcinogenic in several species of laboratory animals. Extreme care should be taken in handling these compounds. The exhaust of the TEA detector should be vented properly in order to reduce exposure to possible excess ozone (03).
5 Apparatus and Equipment
- 250 mL Round-bottom flask with ground glass joint.
- Equipment needed to prepare tobacco for testings specified in T-402.
- Volumetric Flasks 5,10,50,100 mL.
- Aluminum foil.
- Wrist action shaker.
- Glass Pasteur Pipettes.
- Zymark TurboVap II Concentrator equipped with 200 mL Tubes with Graduated 1 mL stem or equivalent.
- Thermal Energy Analyzer (Thermo-Electron Corp.) interfaced to GC or equivalent.
- Gas chromatograph, equipped with temperature programmable injector, electronic flow control, and data handling software.
- GC column, 30 m×0.32 mm×3.0 µm DB-1 fused silica capillary column or equivalent.
- Varian Chem Elut CE20100 Adsorbent tube or equivalent.
- Non-ultra violet (UV) lighting.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade in quality.
- Dichloromethane (DCM) - Distilled in Glass (DIG) or equivalent.
- Acetone - Analytical Grade - DIG or equivalent.
- Sodium sulfate - Anhydrous.
- Basic Alumina.
- Citric Acid (Anhydrous).
- L-Ascorbic acid.
- Sodium Phosphate Dibasic.
- Methanol - DIG or equivalent.
- Type I water (as per ASTM D1193).
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
8 Preparation of Solution
- 1:1 Acetone:Dichloromethane solution
- Mix Acetone and Dichloromethane in a 1:1 v/v basis.
- Citrate-Phosphate Buffer containing L-Ascorbic Acid
- Prepare a 1 L aqueous solution containing :
- 55 mM Citric Acid.
- 90 mM Sodium Phosphate Dibasic.
- 20 mM L-Ascorbic Acid.
Note: pH of the solution must be between 4.3 and 4.5 or it must be remade.
- Prepare a 1 L aqueous solution containing :
9 Preparation of Standards
- N-nitrosoguvacoline (NG) internal standard (ISTD)
- Prepare a solution at 5000 ng/mL in dichloromethane.
- TSNA mixed standard solution
- Prepare a mixed standard dilution stock solution of NNN, NAT, NAB and NNK in dichloromethane at the following range of concentrations:
NNK at 3000 ng/mL.
NNN and NAT at 1500 ng/mL.
NAB at 500 ng/mL.
Note: Concentrated solutions are stable for approximately six months if stored at -20 °C.
- Prepare a mixed standard dilution stock solution of NNN, NAT, NAB and NNK in dichloromethane at the following range of concentrations:
- Build a calibration ranging from approximately 20 ng/mL (for NAB) to 2000 ng/mL (for NNK) containing NG as an internal standard at 500 ng/mL in each of the standards.
Note: Individual calibration stocks are stable for two months if stored at -20 °C.
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Homogenizing of Whole Tobacco
- Cigarette tobacco (or little cigar, fine-cut and, chewing tobacco) is removed from its packaging to make a composite sample.
- Prepare tobacco as specified in T-402.
- A separate 3 g portion of the homogenized tobacco is to be analyzed for moisture in order to present results on a "dry matter" basis.
12 Sample Analysis
- Extraction of Whole Tobacco
- Non-UV lighting shall be used in the room(s) in which this analysis is conducted.
- Accurately weigh 1 g of the whole tobacco into a 250 mL round bottom flask covered with aluminum foil.
- Add 500 µL of NG (ISTD) solution.
- Add 50 mL of Citrate-Phosphate Buffer containing L-Ascorbic acid.
- Place on wrist action shaker for 60 minutes to totally saturate and extract the tobacco.
- Sample Clean-up
- Cut the tip (to allow flow) of the Chem-Elut CE20100 column and place a 200 mL TurboVap tube beneath it.
- Pour the entire extract from the round bottom flask into the CE20100 tube.
- Rinse the round bottom flask with 2×5 mL additions of the citrate-phosphate buffer and add to the Chem-Elut column.
- Wait five minutes to allow the packing to absorb the aqueous solution.
- Rinse the round bottom flask with 150 mL of dichloromethane and add to the CE20100 tube.
- Repeat 12.2.5 with an additional 150 mL of dichloromethane once the first 150 mL has passed through the column and is dripping at a rate of less than 1 drop/second.
- Continue to collect the extract until 250 mL has been collected.
Note: This will require the collection of the TSNA fraction into a second tube, which will be combined with the first tube after some evaporation. - Place the sample into the Turbo evaporator set at 38°C and 10 psi nitrogen.
- Concentrate samples to 3.5 mL (approximately 45 minutes).
- Transfer the concentrate to a 5 mL volumetric flask and make to volume with DCM using DCM washings of the TurboVap tube.
- Transfer the sample to an amber autosampler vial with Teflon-lined septa for GC analysis.
- GC-TEA Operating Conditions
- Carrier flow rate (He): 2.8 mL/minute using electronic flow control (velocity = 60 cm/second).
- Injector temperature: Programmable 35 to 220 °C.
- Oven temperature: Programmed 50 to 170 to 212 °C.
- TEA interface temperature: 240 °C.
- TEA furnace temperature: 500-525°C (dependent on analyzer sensitivity).
- Analysis Run Time: 35 minutes.
- GC-TEA Calibration
- Inject 1.5 µL of the TSNA mixed standard solution and determine peak areas for the four components.
- TSNA Determination
- Inject 1.5 µL of the sample concentrate (11.3.11) and determine areas of the peaks having retention times corresponding to NNN, NAT, NAB and NNK.
- Calculations
- The content, m (ng/g), of a given TSNA is obtained from:
m (ng/g) = CVs/N.
where
C = analytical Concentration determined by ISTD calibration of given TSNA.
Vs = final volume of concentrate.
N = the weight (in g) of tobacco extracted.
- The content, m (ng/g), of a given TSNA is obtained from:
- In order to convert the result to ng/g on a "dry matter basis" , one must correct for moisture by:
m (ng/g)dry matter = m (ng/g) as is / (1- (% Moisture/100)).
where the % Moisture is determined from the same sample "as received" after batch processing.
13 Quality Control
- Typical chromatogram
- See Appendix.
- Method Detection Limits (MDL) / Limit Of Quantitation (LOQ)
- The method detection limit (MDL) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The MDL is calculated as three times the standard deviation of these determinations.
- The MDL (on a ng/g basis) can be calculated by multiplying the determined MDL (ng/mL basis) by the final volume of the extract and dividing this by the weight of tobacco used in the extraction.
- The MDL (on a ng/g basis) can be enhanced by modifying the final dilution volumes used for extraction and clean-up in the procedure.
- The practical limit of quantitation (LOQ) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The LOQ is calculated as 10 times the standard deviation of these determinations.
- The LOQ (on a ng/g basis) can be calculated by multiplying the determined LOQ (ng/mL basis) by the final volume of the extract and dividing this by the weight of tobacco used in the extraction.
- The effect of modifying the final dilution volumes used for extraction and clean-up in the procedure on the LOQ is the same as for the MDL.
14 References
- Adams, J.D., Brunnemann, K.D. & Hoffmann, D., 1983. Chemical studies on tobacco smoke. LXXV. Rapid method for the analysis of tobacco-specific N-nitrosamines by gas-liquid chromatography with a thermal energy analyser. J. Chromatogr., 256, p. 347-351
- Hecht, S.S., Adams, J.D. & Hoffmann, D., 1983. Tobacco-specific nitrosamines in tobacco and tobacco smoke. In: Preussmann, R., O'Neill, I.K., Eisenbrand, G., Spiegelhalder, B. & Bartsch, H., eds, Environmental Carcinogens- Selected Methods of Analysis, Vol. 6, N-Nitroso Compounds (IARC Scientific Publications No. 45), Lyon, International Agency for Research on Cancer, p. 93-101
- Hoffmann, D., Adams, J.D., Brunnemann, K. D. & Hecht, S. S., 1979. Assessment of tobacco-specific N-nitrosamines in tobacco products. Cancer Res., 39, p. 2505-2509.
- Spiegelhalder, B., Kubacki, S.J., and Fischer, S., 1989. "A Method for the Determination of Tobacco-specific Nitrosamines (TSNA), Nitrate and Nitrite in Tobacco Leaves and Processed Tobacco". Beitrage zur Tabakforschung International 14, p. 135-143.
- Risner, Charles H., and Wendelboe, Fred N., 1994. Quantification of Tobacco Specific Nitrosamines in Tobacco, Tob. Sci. 38, p.1-6, 1994.
- Protocol to Measure the Quantity of Nicotine Contained in Smokeless Tobacco Products Manufactured, Imported, or Packaged in the United States, 1997. Federal Registrar, Vol.62, No.85, Friday, May 2, 1997.
Appendix
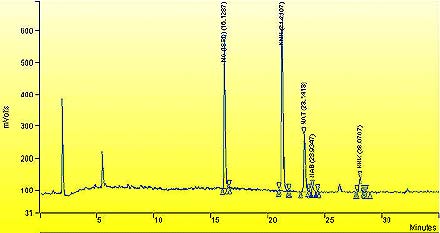
An example chromatogram of the extract of whole tobacco for 1R4F
The following figure is a sample chromatogram of extract of whole tobacco for 1R4F. The voltage in mVolts is plotted against time in minutes. The detected peaks include N-nitrosoguvacoline (NG-internal standard) at 16.1287 min , N-nitrosonornicotine (NNN) at 21.2167 min, N-nitrosoanatabine (NAT) at 23.1413 min, N-nitrosoanabasine (NAB) at 23.9347 min and 4-(N-nitrosomethylamino)- I -(3-pyridyl)- 1 -butanone (NNK) at 28.0767 min.