Determination of Benzo[A]pyrene in Mainstream Tobacco Smoke
Health Canada
T-103 December 31, 1999
Table of Contents
- Scope of Application
- Normative references
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- Modifications for Intense Smoking Conditions
- Reference
- Appendices
1 Scope of Applications
- Applicable to the quantitation of benzo[a]pyrene (B[a]P) content in the total particulate matter (TPM) of mainstream (MS) tobacco smoke by reversed phase high performance liquid chromatography (HPLC) via fluorescence detection.
2 Normative References
- American Society for Testing and Materials (ASTM) D1193-77 - Standard Specification for Reagent Water, Version 1977.
- Health Canada Test Method T-115 - Determination of the Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
- Methods of Sampling and Testing Tobacco: Determination of Benzo[a]pyrene in Total Particulate Matter of Tobacco Smoke. National Standard of Canada, Canadian General Standards Board CAN/CGSB-176.2 No. 1-96, March 1996.
3 Definitions
- Refer to T-115 for definitions of terms used in this document..
4 Method Summary
- Tobacco product is smoked as specified in T-115.
- Total particulate matter, collected on a glass fibre filter disc (pad), is extracted with a sufficient amount of cyclohexane to create an extract with a concentration of approximately 1 mg of wet particulate matter/mL extract.
- A portion of this solution is filtered through a 0.45 µm PTFE filter to a 7 mL glass vial for storage. A 2 mL aliquot of the extract is passed through a 1 g (6 mL) silica cartridge and 360 mg NH2 plus cartridge in series.
- The B[a]P is eluted with hexane, evaporated under a constant stream of nitrogen to dryness, and reconstituted to a 1 mL volume with acetonitrile.
- The sample is subjected to reversed phase high performance liquid chromatography (HPLC) and quantitated via fluorescence detection.
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed to perform smoking analyses as specified in T-115.
- Equipment needed to perform conditioning of tobacco product as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Supelco Visi-Prep Solid Phase Extraction (SPE) unit (24 cartridge unit) or equivalent.
- 2 mL glass pipettes.
- Brinkman Dispensette (10-50 mL) or equivalent.
- Micro-pipettes (10, 50, 500, 1000 µL).
- 125 mL and/or 250 mL Erlenmeyer (or round bottom) flasks with ground glass joints.
- Zymark TurboVap II Concentrator or equivalent.
- 2 L volumetric flask.
- Wrist-action shaker.
- Glass transfer pipettes.
- Laboratory mixer.
- Merck 250 × 4 mm, RP-18e, 5 µm packing HPLC column or equivalent.
- Lichrocart 4-4 Lichrosphere 100 RP-18 endcapped 5 mm guard column or equivalent.
- Analytical balance, capable of measuring to four decimal places.
- High Performance Liquid Chromatograph consisting of:
- Autosampler.
- Tertiary pump.
- Fluorescence Detector.
- Data collection system.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade quality.
- Benzo[a]pyrene (B[a]P).
- Cyclohexane.
- Hexane.
- Acetonitrile.
- Methanol.
- Isopropanol (IPA).
- Acetone.
- Anhydrous Sodium Sulphate.
- Tetrahydrofuran (THF).
- 16 × 125 mm culture tubes (20 mL).
- Glass fibre filter disc (pad) and holders (45 mm).
- Disposable 5 cc syringe.
- Autosampler vials, caps and teflon lined septa.
- Pasteur Pipettes.
- 1 g silica Sep-Pak cartridges (6 mL capacity).
- 360 mg NH2 Plus Sep-Pak cartridge.
- 7 mL screw top vials with aluminum lined cap.
- 13 mm 0.45 µm PTFE disposable filters.
- Type I water as per ASTM D1193.
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
8 Preparation of Solutions
- Prepare solutions required for analysis, as specified in T-115, in accordance with good laboratory practice..
9 Preparation of Standards
- Preparation of Spiking Solution for Lab Fortified Samples
- Primary (1°) B[a]P Stock: Dissolve 10 mg B[a]P into 50 mL Cyclohexane.
- Secondary (2°) Stock: Pipette 50 µL of 1o Stock into 50 mL Cyclohexane.
- A 10 µL volume of spiking solution is added to a second 2 mL aliquot of a control brand cigarette extract solution prior to solvent substitution (14.2.4 to 14.2.8) and clean-up through the SPE cartridges to make the Laboratory
Fortified Matrix (LFM*). Another 10µL volume of spiking solution is added to a second 2 mL aliquot of the Laboratory Reagent Blank (LRB*) prior to solvent substitution and clean-up through
the SPE cartridges to make the Laboratory Fortified Blank (LFB*). The spiking analytical concentration is approximately 2 ng/mL (dependent on stock concentration).
*See section on Quality Control for explanations of these initialisms.
- Preparation of Working Standards
- Primary (1°) B[a]P Stock: Dissolve 10 mg B[a]P into 50 mL Acetonitrile.
- Secondary (2°) Stock: Pipette 100 µl of 1 o Stock into 50 mL Acetonitrile.
The following table summarizes the set of working standards made, the volume of each secondary stock, as well as the corresponding final volume and concentration of each solution.
Working standards Standard # Volume of Secondary 2o stock (µL) Final Volume (mL) Concentration [ng/mL] 1 40 25 0.6400 2 175 25 2.800 3 350 25 5.600 4 600 25 9.600 5 900 25 14.4 6 2 mL of Std 1 10 0.1280 7 4 mL of Std 1 10 0.2560
All weights, volumes, and purity must be recorded and used to accurately calculate the standard concentrations. These concentrations are only representations of standards used in a calibration curve.
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars are to be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115.
13 Sample Generation
- Cigarettes shall be smoked and TPM collected as specified in T-115.
Note: Five cigarettes per observation are to be smoked under standard conditions. For other tobacco products, select a number such that breakthrough does not occur.
Note: Samples may be stored at -20 °C for up to 10 days prior to extraction with cyclohexane.
14 Sample Analysis
- Extraction of Filter Pads
- Remove the mainstream pad from its holder, folding it into quarters and wiping the inside of the holder with the clean side of the pad.
- Transfer the pad into a round bottom flask.
- Pipette a volume of cyclohexane (minimum amount is 30 mL) into the round bottom flask that is numerically equivalent to the total TPM in mg (rounded to the nearest 10 mL) yielding a concentration of approximately 1 mg
TPM/mL of cyclohexane. Record the volume of the cyclohexane used to extract the pad.
- Example: 1. If TPM = 70 mg (total), then cyclohexane volume = 70 mL.
- Example: 2. If TPM = 83 mg (total), then cyclohexane volume = 80 mL.
- Example: 3. If TPM = 57 mg (total), then cyclohexane volume = 60 mL.
- Shake the round bottom flask containing the mainstream pad and cyclohexane vigorously on a wrist action shaker for 30 minutes, until there appears to be a homogeneous solution and there is no longer any evidence of localized colour on the pad.
- Place the flasks in the dark until they are ready for further processing.
- Sample Clean-up
- Filter approximately an 8 mL portion of the cyclohexane extract through a 0.45 µm PTFE disposable organic filter into a 7 mL vial with foil lined cap. Samples may be stored at 4 °C at this point if adequate headspace is left.
- Pre-condition both (the silica and the NH2 plus) cartridges using hexane as recommended by the manufacturer.
Note: All air has to be removed from the packing thus exposing sorbent material to the hexane. - Place the pre-conditioned cartridges on the Visi-prep unit. Add about 1 g anhydrous sodium sulfate to the silica cartridge and wash the cartridges with 10 mL hexane allowing the hexane to flow through the cartridge by gravity.
- Once the extract has reached room temperature, pipette a 2 mL aliquot of the extract onto the packing of the Silica cartridge.
- Allow the cyclohexane to pass through the SPE cartridges (by gravity) at a rate of approximately one drop/second. Discard the eluant.
- Pipette 4 mL of hexane to the cartridge allowing the eluant to gravimetrically pass through the cartridge. Discard the eluant.
- Place 20 mL disposable glass culture tubes beneath each of the cartridges.
- Gravimetrically elute the B[a]P from the cartridges with 4 × 4 mL additions of hexane.
- Add 1 mL of THF to each tube.
- Place the tubes containing the 17 mL of collected eluant into the Zymark Turbo-vap.
Note: Conditions are to be set at 40 °C with a Nitrogen pressure of 7.5 psi. - Evaporate the samples to complete dryness.
Note: This will require an initial 20 minute period in which the nitrogen pressure may be slowly increased to a maximum of 10 psi in a manner such as to prevent any loss of sample from splattering. - Remove samples that are completely dry. If some samples are not completely dry, process the samples in additional five minutes intervals.
- Pipette 1000 µL of Acetonitrile into each of the dried tubes to dissolve the analyte and any residue that may be present.
- Vortex the sample at high speed for approximately 15 seconds.
- Using a glass transfer pipette, wash down the sides of the tube five times with the sample, and transfer the sample to an autosampler vial with a screw cap and Teflon faced septa.
- The samples are ready for HPLC analysis and may be stored at 4 °C until they are analyzed.
- Instrument Analysis - Reversed Phase High Performance Liquid Chromatography (HPLC) Analysis
- Jasco Fluorescence Detector Conditions
Excitation Wavelength: 365 nm.
Emission Wavelength: 425 nm.
Gain: × 1000. Attenuation: 32.
Note: A different manufacturer's fluorescence detector may need to be programmed differently to maintain the full calibration range. A slight change in excitation and emission wavelength may be required dependent on manufacturer (i.e. 366 and 424 for the wavelengths). - Autosampler : Injection Volume
Analyze using a 50 µL sample loop and set the parameter for injection volume at 75 µL to ensure a thorough flushing of the sample loop with the sample. - Mobile Phase / Gradient Conditions (Tertiary Gradient System)
Solvent A: 55:45 Acetonitrile: 1 % isopropyl alcohol in Type I water (degassed and filtered using a 0.45 µm nylon filter).
Solvent B: Methanol.
Solvent C: Acetonitrile.
Flow: 1.5 mL/minute.
Gradient: Adjustments to the gradient may be required depending on column conditions and resolution of analyte.
The following table summarizes the percent separation of solvents A, B and C at various times using high performance liquid chromatography. Samples were collected at time 0 and at various intervals from 20 to 35 minutes from starting point.
Equilibration Time: 8.00 minutes.Mobile Phase / Gradient Conditions Time (minutes) Composition % A % B % C 0.00 55 0 45 20.00 75 0 25 25.01 100 0 0 28.00 100 0 0 30.01 0 100 0 32.00 0 100 0 34.01 100 0 0 35.00 100 0 0 35.00 Method End Action: Equilibrate
- Jasco Fluorescence Detector Conditions
- Calculations
- Determination of Response Factor
- An initial calibration is performed by running prepared standards from high concentration to low concentration (injecting the very first standard a minimum of two times until the response and retention time are constant).
- A calibration curve is prepared by plotting the concentration of B[a]P in the standard vs. the peak height response from the fluorescence detector.
- The Response factor is the slope of the line as determined by linear regression (Height counts/unit concentration).
- Determination of B[a]P Delivery [ng/cig]
B[a]P [ng/cig] = [Peak Height × Volume Extractant (mL) × Final Volume (mL)] / [Response Factor × # Cigarettes Smoked × Aliquot Volume (mL)]
where the aliquot volume (mL) is the volume transferred to the Sep-pak cartridges correcting for any potential previous dilutions in the solvent substitution step. The Response Factor is determined from the calibration curve.
- Determination of Response Factor
15 Quality Control
- Typical Chromatogram
- See Appendix 1a and 1b .
- Typical Control Parameters
- Each set analysis should contain at least one of each of the following per smoking run (20 port smoking):
- Laboratory Reagent Blank (LRB): to determine background contamination from solutions, glassware, or materials used in the analysis process.
- Laboratory Fortified Blank (LFB): to determine whether there is any loss of analyte as a result of the analysis process.
- Laboratory Fortified Matrix (LFM): by spiking one of the control brand cigarettes: to determine whether there is any loss of analyte as a result of the analysis process and to determine potential matrix effects.
- Reference Sample: to determine the inter-experimental reproducibility of the entire method of analysis
- Duplicate Sample: to determine the reproducibility of the procedure within the same experiment or batch on analysis.
- Each set analysis should contain at least one of each of the following per smoking run (20 port smoking):
- Recoveries and Levels of Contamination
- Typical recoveries of Laboratory Fortified Blanks (LFB) and Laboratory Fortified Matrix (LFM) samples range from 85 - 110 % when a spiked solution (or sample) is carried out through the entire extraction process.
- Recoveries lower than 85 % indicate either an insufficient elution of B[a]P from the solid phase extraction cartridges or a change in response factor (RF) of the fluorescence detector. A change in RF must first be investigated before re-processing of samples is initiated.
- Typical Laboratory Reagent Blanks (LRB) range from a calculated value of 0 - 0.3 ng/cigarette. Contamination of this type is usually associated with contamination of the filter pad during conditioning or an inadequate cleaning of glassware.
- Method Detection Limits (MDL) / Limit of Quantitation (LOQ)
- The method detection limit (MDL) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The MDL is calculated as three times the standard deviation of these determinations.
Note: The MDL (on a ng/cig basis) can be manipulated by modifying the number of cigarettes smoked and the volumes used for extraction and clean-up in the procedure. - The practical limit of quantitation (LOQ) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The LOQ is calculated as 10 times the standard deviation of these determinations.
- The method detection limit (MDL) is determined by analyzing the lowest standard level a minimum of 10 times as an unknown over several days. The MDL is calculated as three times the standard deviation of these determinations.
- Stability of Reagents and Samples
- Analytical stocks and standard should be stored at -20 °C.
- Stock standards and stock spike solutions remain stable for up to six months. Although there is no loss of analyte, evaporation (loss) of solvent may be an issue.
- Analytical run standards should be freshly prepared every two months.
- Samples are stable at 4 °C for three weeks after extraction.
16 Modifications for Intense Smoking Conditions
- Under intense smoking conditions, the number of cigarettes per observation is reduced to two.
17 Reference
- Dumont, J., Larocque-Lazure, F., and Iorio, C. An Alternative Isolation Procedure for the Subsequent Determination of Benzo[a]pyrene in Total Particulate Matter of Cigarette Smoke, Journal of Chromatographic Science, Vol. 31, September 1993, p. 371-374.
- Tomkins, B.A.; Jenkins, R.A.; Griest, W.H.; Reagen, R.R. Liquid Chromatographic Determination of Benzo[a]pyrene in Total Particulate Matter of Cigarette Smoke, J. Assoc. Off. Anal. Chem., Vol. 68, 5, 1985, p. 935-940.
Appendices
Appendix 1a: Typical Chromatogram
The following figure displays a typical chromatogram, with an overlay of a standard, a high tar reference cigarette and a low tar reference cigarette.
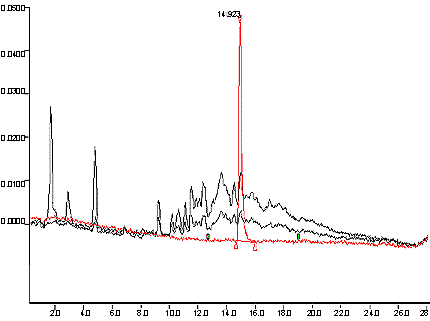
An overlay of a standard, a high tar reference cigarette and a low tar reference cigarette.
Appendix 1b: Typical Chromatogram
The following figure is an expansion of figure 1a to show the integration of the baseline for the real sample.
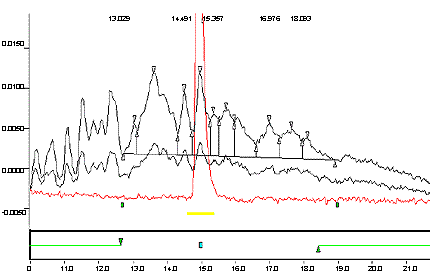
An expanded view of Appendix 1a to show the integrating baseline of a true sample.