Determination of Selected Carbonyls in Mainstream Tobacco Smoke
Health Canada
T-104 December 31, 1999
Table of Contents
- Scope of Application
- Normative references
- Definitions
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware
- Preparation of Solutions
- Preparation of Standards
- Sampling
- Tobacco Product Preparation
- Smoking Machine Preparation
- Sample Generation
- Sample Analysis
- Quality Control
- Modifications for Intense Smoking Conditions
- Reference
- Appendices
1 Scope of Applications
- Applicable to the trapping and quantitation of volatile carbonyl compounds (as their 2,4-dinitrophenylhydrazones [DNPH]) in the vapour phase of mainstream tobacco smoke. This is applicable to the carbonyl compounds extracted from the mainstream vapour phase and quantitated from the DNPH trapping solution only. The method is applicable to tobacco smoke generated by both cigarettes and cigars.
2 Normative References
- American Society for Testing and Materials (ASTM) D1193-77 - Standard Specification for Reagent Water, Version 1977.
- Health Canada Test Method T-115 -Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, 1999-12-31.
3 Definitions
- Refer to T-115 for definitions of terms used in this document..
4 Summary of Method
- Tobacco product is smoked on alternate ports of a standard 20 port linear smoking machine that has been fitted with Drechsel-type bottles or traps with fritted impingers.
- The unfiltered mainstream tobacco smoke is scrubbed of volatile carbonyls by passing each puff through an impinger into a trap containing 80 mL of an acidified solution of 2,4-dinitrophenylhydrazine in acetonitrile.
- An aliquot of the reacted DNPH-smoke extract is then syringe-filtered, diluted with 1 % trizma base in aqueous acetonitrile.
- The samples are subjected to reverse phase high performance liquid chromatography (HPLC) and quantitated via ultra violet detection
Note: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that could potentially be hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with any existing applicable regulatory requirements prior to its use.
The sample preparation and analysis should be completed in one day and the solvent waste generated by the HPLC must be stored for disposal by a registered chemical-recycling agency.
5 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in T-115.
- Equipment needed to perform marking for butt length as specified in T-115.
- Equipment needed to perform smoking of tobacco products as specified in T- 115.
- Wrist action shaker.
- 150 mL Erlenmeyer flasks with ground glass stoppers.
- 12 glass cigarette holders (6.5 cm in length and 8.0 mm internal diameter).
- 12 glass Drechsel Type traps (capacity 250 mL) with fritted extra coarse impingers.
- Nalgene Tubing 1/4" ID × 3/8" OD.
- Analytical balance, capable of measuring to four decimal places.
- Volumetric flasks 10 mL, 25 mL, 1 L, and 2 L.
- Glass micropipettes - assorted volumes (100, 150, 300, 400, 500, 800, 1000, and 2000 µL).
- Glass transfer pipettes - 1, 2, 5, 6, 7, 8, and 20 mL.
- Glass graduated measuring cylinders 25 mL and 50 mL.
- Hot Plate/Stirrer.
- PC controlled High Performance Liquid Chromatography System consisting of:
- Tertiary gradient pump.
- Autosampler with 50 µL sampling loop.
- UV Detector.
- Data Collection System.
- Column: Merck Lichrosphere 250 × 4 mm, 100, RP 18e (5 µm) or equivalent.
- Disposable Guard Column: Lichrocart 4 × 4 mm, Lichrosphere RP 18e (5 µm) or equivalent.
- Vacuum filter.
- Amber bottles 1 L and 4 L.
- Dessicator.
6 Reagents and Supplies
Note: All reagents shall be, at the least, recognized as analytical reagent grade quality.
- Methanol - Distilled in glass (DIG).
- Acetonitrile (MeCN) - DIG.
- Isopropanol (IPA) - DIG.
- Ethyl Acetate - DIG.
- Tetrahydrofuran (THF) - DIG.
- Reagent Alcohol - HPLC Grade.
- Perchloric Acid (60 %).
- Hydrochloric Acid (35 %).
- Sulphuric Acid, Concentrated (H2SO4).
- Type I water as per ASTM D1193.
- Formaldehyde Solution - 37-41 % (w/v).
- Acetaldehyde > 99 % purity.
- Acetone - DIG.
- Acrolein > 99 % purity.
- Propionaldehyde > 97 % purity.
- Crotonaldehyde > 99+ % purity.
- Methyl Ethyl Ketone > 99+ % purity.
- Isobutyraldehyde > 99 % purity.
- Butyraldehyde > 99+ % purity.
- Trizma Base.
- 2,4-dinitrophenylhydrazine.
- Syringe filter - 0.45 µm PVDF or equivalent.
- Disposable syringes - 5 mL.
- Disposable glass Pasteur pipettes.
- Rubber bulbs.
- Autosampler Vials (amber), caps and Teflon faced septa.
- Masking Tape.
- Parafilm® (or equivalent).
- Helium (UHP).
7 Preparation of Glassware
- Glassware should be cleaned and dried in such a manner to ensure that contamination from glassware does not occur.
- It is extremely important that all possible sources of contamination are removed from the work area e.g. acetone solvent wash bottles.
8 Preparation of Solutions
- Preparation of DNPH Solution
- Weigh 6.792 g (24.0 mmol) of commercially available 2,4 dinitrophenylhydrazine. Add to 1 L of fresh acetonitrile in a 2 L volumetric flask. Dissolve DNPH by alternating: gently swirling and warming the flask. Make sure there are no crystals remaining before proceeding. (Warning! Do not sonicate.)
- After the DNPH is dissolved, add 5.6 mL 60 % perchloric acid with gentle mixing. The solution will turn yellow at this point.
- Dilute to volume with Type I water. The solution will turn to a bright orange upon addition of the water.
- Store the solution in a 4 L amber bottle at room temperature in the dark to reduce the chances of DNPH precipitation. This solution, if properly sealed, will remain stable for one week under these conditions.
- Preparation of Trizma Base Dilution Solution ( 80:20, MeCN:1 % aqueous Trizma )
- Dissolve 2.00 g of Trizma Base in 200 mL of distilled deionized water (Type I water) in a 1 L volumetric flask. Dilute to volume with acetonitrile.
- Store in a 1 L amber bottle with Teflon-lined cap at room temperature. This solution should remain stable for several weeks under these conditions.
9 Preparation of Standards
- Preparation of Dinitrophenylhydrazone derivatised carbonyls
- Dissolve 600 mg commercially available DNPH in 2 mL concentrated H2SO4 in a 50 mL Erlenmeyer flask.
- Stir with a glass rod while adding 3 mL of Type I water (clear solution). Then add 10 mL of reagent alcohol.
- Add the DNPH solution to a solution of the appropriate aldehyde or ketone containing (each as an individual preparation):
120 mg formaldehyde
50 mg acetaldehyde
40 mg acetone
40 mg acrolein
40 mg propionaldehyde
35 mg crotonaldehyde
33 mg methyl ethyl ketone
33 mg butyraldehyde.
Crystallisation generally occurs rapidly. - Filter crystals (hydrazones) using a vacuum filter and rinse the crystals with cold (4 °C) reagent alcohol.
- Recrystallization of hydrazones: Add about 10 mL reagent alcohol to the crystals in a small Erlenmeyer flask, heat and then add 3 mL ethyl acetate dropwise to dissolve crystals. Cool to room temperature.
- Filter crystals under vacuum, rinse with cold (4 °C) reagent alcohol, air dry and then store in vials in desiccator at -20 °C.
- HPLC Calibration Standards and Working Solutions
- Primary (1°) Carbonyl Standards
- Weigh purified hydrazones in the amounts described in Appendix 1(a). Put into individual 25 mL volumetric flasks and dissolve in acetonitrile. Concentration is of the free aldehyde.
- Seal each volumetric flask with parafilm and refrigerate at 4 °C. When properly stored, solutions are stable for up to one year.
- Secondary (2°) Carbonyl Standards
- Pipette predetermined volumes of each primary hydrazone standard into a single 25 mL volumetric flask and dilute up to the mark with acetonitrile.
- Seal volumetric flask with parafilm and refrigerate at 4 °C. Prepare new working standards every 20 days. See Appendix 1(a).
- Carbonyl Working Standards
- Take appropriate volumes (0.050 to 10 mL) of the 2° carbonyl standard and dilute to 10 mL with acetonitrile to give calibration standards with approximate carbonyl concentrations in the ranges noted in Appendix 1(b).
- Transfer to autosampler vials.
- New carbonyl calibration standards should be prepared every 20 days.
- Carbonyl Spiking Solution (see Appendix 1c)
- Pipette predetermined volumes of each primary hydrazone standard into a single 25 mL volumetric flask and dilute up to the mark with acetonitrile.
- Prepare new spiking solution every 20 days.
- Primary (1°) Carbonyl Standards
10 Sampling
- The sampling of tobacco products for the purpose of testing shall be as specified in T-115.
11 Tobacco Product Preparation
- Product shall be conditioned as specified in T-115.
- Cigarettes, cigarette equivalents, bidis, kreteks and cigars shall be marked for butt length as specified in T-115.
- Cigarettes to be smoked under intense smoking conditions shall be prepared as specified in T-115.
12 Smoking Machine Preparation
- Ambient Conditions
- The ambient conditions for smoking shall be as those specified in T-115.
- Machine Conditions
- The machine conditions shall be as those specified in T-115 (with the following modifications as detailed below:)
- It is important to ensure that the mainstream tobacco smoke is characteristic of the test sample before proceeding with the analysis. Because the mainstream total particulate matter (TPM) is not filtered, no filter pad is present; puff count information must then be used to characterise the smoke extract samples and monitor the smoking process.
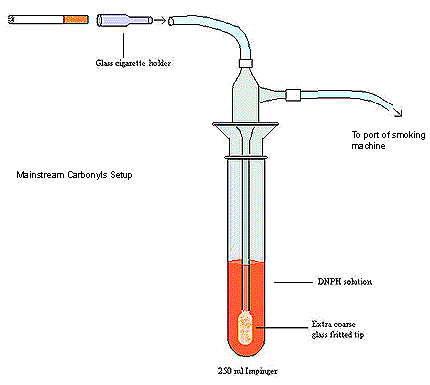
- Assemble the carbonyl mainstream apparatus on 12 alternating ports of the linear smoking machine without using the filter pads and holders.
- Connect the special glass cigarette holder (6.5cm in length and 8.0 mm internal diameter) at the back by Nalgene tubing to the 250 mL impinger and trap. See diagram.
- Check and adjust the puff volume drawn by the smoking machine in all of the 12 ports as per T-115. Volumes are checked at the cigarette end of the port (with fritted impinger and DNPH in line) via the brass restrictor.
- The same impinger can be used to adjust each port before smoking begins. Discard the DNPH solution after puff volumes have been measured and adjusted.
- Add 80 mL of fresh 2,4-DNPH solution to each impinger.
- The machine conditions shall be as those specified in T-115 (with the following modifications as detailed below:)
13 Sample Generation
- Cigarettes (two cigarettes*/observation) shall be smoked per observation as specified in T-115.
*For other tobacco products, select a number such that breakthrough does not occur.
14 Sample Analysis
- Mainstream Smoke Extract Solution
- One run consists of 12 DNPH smoke extract samples. Process 12 samples at a time but not more than two runs or 24 samples per day. Do not smoke more than can be analysed in a 24 hour period.
- Rinse the cigarette holder and the Nalgene tubing with the impinger solution by forcing the impinger solution back up the impinger as far as the glass cigarette holder using positive air pressure and then with negative air pressure until air is forced back through the solution.
- Repeat this rinsing procedure at least three times for each impinger to dissolve any smoke condensate in the gas transfer lines.
- Allow the DNPH smoke extract solution to sit for least five minutes before continuing with sample preparation.
- Pipette 6 mL of 1 % Trizma base solution into a 10 mL volumetric flask.
- Add 4 mL of syringe-filtered DNPH smoke extract to the volumetric flask.
- Mix the volumetric flask well. Transfer a portion of this solution by Pasteur pipette to autosampler vials in duplicate (a and b). (Rinse each vial first with a few drops, fill to minimise head space).
- Cap the vials with Teflon faced septa and stored at 4 °C until analysed.
- Repeat 14.1.5 to 14.1.8 for each smoke extract sample.
- Preparation of Controls and Blanks
- Prepare at least one LRB, LFB, and one LFM per day of activity as follows to demonstrate that interference from the analytical system, glassware, and reagents are not present.
- Laboratory Reagent Blank (LRB)
- Pipette 6 mL of the 1 % Trizma base dilution solution into a 10 mL volumetric flask.
- Add 4 mL of fresh filtered DNPH solution to the volumetric flask. Cap the flask and mix well.
- Transfer to two autosampler vials (a and b), cap and store at 4 °C until ready to analyse.
- Laboratory Fortified Blank (LFB)
- Add 1 mL of the Carbonyl Spiking Solution and 79 mL of DNPH solution to the 250 mL impinger. Mix well.
- Pipette 6 mL of the 1 % Trizma base dilution solution into a 10 mL volumetric flask.
- Add 4 mL of the filtered DNPH/Spiking solution (14.4.1) to the volumetric flask. Cap the flask and mix well.
- Transfer to two autosampler vials (a and b), cap and store at 4 °C until ready to analyse.
- Laboratory Fortified Matrix (LFM)
- Pipette 5 mL of the 1 % Trizma base dilution solution into a 10 mL volumetric flask.
- Add 1 mL of the Carbonyl Spiking Solution to the 10 mL volumetric flask.
- Add 4 mL of filtered DNPH/smoke extract solution from a control brand to the 10 mL volumetric flask. Cap the flask and mix well.
- Transfer to two autosampler vials (a and b), cap and store at 4 °C until ready to analyse.
- Reversed Phase High Performance Liquid Chromatography
- Chromatographic Conditions
- Column Temperature: 30 °C.
- Injection volume: 20 µL.
- UV detection at 365 nm.
- Mobile Phase: Reagents
Solvent A: Prepare 2 L of 30 % Acetonitrile, 10 % THF, 1 % IPA in Type I water, filter and degas. (UHP Helium sparged).
Solvent B: Prepare 2 L of 65 % Acetonitrile, 1 % THF, 1 % IPA in Type I water, filter and degas. (UHP Helium sparged).
Solvent C: Acetonitrile (UHP Helium sparged). - Sample Wash: Solvent A.
- Mobile Phase: Gradient.
Flowrate Time (minutes) Composition 1.5 mL/minute % A % B % C 0.0 100 0 0 8.0 70 30 0 20.0 47 53 0 27.0 0 100 0 30.0 0 0 100 32.0 0 0 100 34.0 95 5 0 Method End Action: 100 0 0 (Equilibrate 10 minutes)
- Sample vials are loaded onto the autosampler such that every 8th vial is a standard solution and in such quantities that the total analysis time (14.1 - 14.6) does not exceed 24 hours.
- Inject 20 µL of vial (a) of each sample onto the HPLC column and analyze as per the chromatographic conditions listed in 14.6.1. Vial b is the backup sample in the event of a problem.
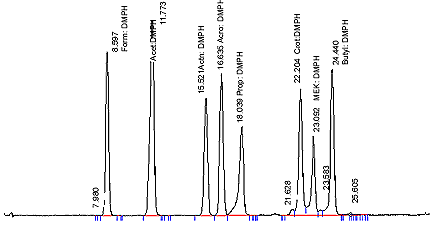
- Elution pattern should be similar to Figure 1.
- Chromatographic Conditions
- Calculations
- Construct a Calibration Curve:
- Twenty µL of each calibration standard is injected onto the HPLC column and analysed as per the chromatographic conditions listed in 12.7. Do in duplicate. Elution pattern should be similar to Figure 2.
- Determination of Response Factor
- A calibration curve for each individual carbonyl is prepared by plotting the concentration of the standards versus their respective peak areas.
- Response factors are calculated for each individual carbonyl compound from the calibration curves.
- Sample Quantification
- The amount of the various carbonyl compounds in smoke samples is quantified by the external standard method. The identification of peaks is by comparison of retention times with standards, and the spiking of smoke samples.
- Carbonyl concentrations are reported in (µg/mL) by the software.
- Determination of Mainstream Carbonyl Deliveries in [µg/cigarette]
e.g. Carbonyl [µg/cigarette] =[ Peak Area/ Resp. Factor] × [DF/ No. of Cigarettes]
where DF is the dilution factor. The response factor shall be determined from the calibration curve.
- Construct a Calibration Curve:
15 Quality Control
- Typical Chromatogram
- See Figures1, 2. .
- Recoveries and Levels of Contamination
- Each analytical run of test cigarettes should include:
A Laboratory Reagent Blank (LRB) to evaluate the extent of any interference due to glassware, trapping reagents, glass fibre filter discs (pads), and analyzer effects.
A Laboratory Fortified Matrix (LFM) to evaluate the extent of potential analyte loss.
A standard run as a sample to verify the calculation process and validate the calibration.
- Each analytical run of test cigarettes should include:
- Method Detection Limit (MDL) and Limit of Quantitation
- Method Detection Limit (MDL)
- The method detection limit is determined by analysing the lowest level standard at least 10 times as an unknown over several days. The MDL is then calculated as three times the standard deviation of these determinations.
- Limit of Quantitation (LOQ)
- The limit of quantification is determined by analysing the lowest level standard at least 10 times as an unknown over several days. The LOQ is then calculated as ten times the standard deviation of these determinations.
- Method Detection Limit (MDL)
- Stability of Reagents and Supplies
- All primary Carbonyl standards are prepared as required.
- All work standards are to be prepared every 20 days.
- All samples are to be analysed as soon as they are prepared and within four hours of the cigarettes being smoked.
16 Modifications for Intense Smoking Conditions
- No modifications for intense smoking are necessary.
17 Reference
- Houlgate, P. R., Dhingra, K. S., Nash, J. S., and Evans, W. H., 1989: Determination of Formaldehyde and Acetaldehyde in Mainstream Cigarette Smoke by high-performance Liquid Chromatography; Analyst 114, p. 355-360.
- Manning, D.L., Maskerinec, M.P., Jenkins, R.A., and Marshall, A.H. "High Performance Liquid Chromatographic Determinations of Selected Gas Phase Carbonyls in Tobacco Smoke" Journal of Assoc of Anal Chem ., 66, p. 8-12.
Appendices
Appendix 1 - Typical Calibration Standards
(a): Stock Standards
This table provides a summary of the primary and secondary stock standards for target analytes, including formaldehyde, acetaldehyde, acetone, acrolein, propionaldehyde, crotonaldehyde, MEK, butyraldehyde. The table contains information regarding weight with hydrazone, weight of carbonyl, the weight, purity, volume of the sample, as well as the stock concentrations for the primary standards. The table also summarizes information regarding the diluted secondary standards, including the volume of initial primary standards and the final volume and concentrations.
| Carbonyl Hydrazone |
Primary Standard | Secondary Standard | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Formula Wt Hydrazone |
Formula Wt Carbonyl |
Weight (mg) |
Purity % |
Volume (mL) |
Stock [µg/mL] |
Vol. (mL) Primary Stock |
Dilute to Vol. (mL) |
Stock [µg/mL] |
|
| Formaldehyde | 211.2 | 30.03 | 39.72 | 100 | 25 | 225.879 | 0.5 | 25 | 4.51758 |
| Acetaldehyde | 225.14 | 44.05 | 53.9 | 100 | 25 | 421.834 | 1.0 | 25 | 16.87338 |
| Acetone | 239.17 | 58.08 | 31.2 | 100 | 25 | 303.064 | 0.75 | 25 | 9.09192 |
| Acrolein | 237.15 | 56.06 | 32.27 | 100 | 25 | 305.133 | 0.5 | 25 | 6.10266 |
| Propionaldehyde | 239.17 | 58.08 | 31.18 | 100 | 25 | 302.87 | 0.5 | 25 | 6.0574 |
| Crotonaldehyde | 251.18 | 70.09 | 27.37 | 100 | 25 | 305.496 | 0.5 | 25 | 6.10992 |
| MEK | 253.2 | 72.11 | 27.43 | 100 | 25 | 312.477 | 0.5 | 25 | 6.24953 |
| Butyraldehyde | 253.2 | 72.11 | 23.28 | 100 | 25 | 265.201 | 0.5 | 25 | 5.30402 |
*In a single 25 ml volumetric flask and made up to volume with acetonitrile.
(b): Carbonyl Working Standards
This table provides a summary of the carbonyl working standards, with concentrations for 5,20,40,80,200,400,700 and 1000 µg/mL standards. The corresponding concentration of the target analytes from Table 1a is specified.
| 5 | 20 | 40 | 80 | 200 | 400 | 700 | 1000 | |
|---|---|---|---|---|---|---|---|---|
| Vol. (ml) W/S | 0.050 | 0.200 | 0.400 | 0.800 | 2.000 | 4.000 | 7.000 | 10.000 |
| Carbonyl | [μg/mL] | [μg/mL] | [μg/mL] | [μg/mL] | [μg/mL] | [μg/mL] | [μg/mL] | [μg/mL] |
| Formaldehyde | 0.0226 | 0.0904 | 0.1807 | 0.3614 | 0.9035 | 1.8070 | 3.1623 | 4.5176 |
| Acetaldehyde | 0.0844 | 0.3375 | 0.6749 | 1.3499 | 3.3747 | 6.7494 | 11.8114 | 16.8734 |
| Acetone | 0.0455 | 0.1818 | 0.3637 | 0.7274 | 1.8184 | 3.6368 | 6.3643 | 9.0919 |
| Acrolein | 0.0305 | 0.1221 | 0.2441 | 0.4882 | 1.2205 | 2.4411 | 4.2719 | 6.1027 |
| Propionaldehyde | 0.0303 | 0.1211 | 0.2423 | 0.4846 | 1.2115 | 2.4230 | 4.2402 | 6.0574 |
| Crotonaldehyde | 0.0305 | 0.1222 | 0.2444 | 0.4888 | 1.2220 | 2.4440 | 4.2769 | 6.1099 |
| MEK | 0.0312 | 0.1250 | 0.2500 | 0.5000 | 1.2499 | 2.4998 | 4.3747 | 6.2495 |
| Butyraldehyde | 0.0265 | 0.1061 | 0.2122 | 0.4243 | 1.0608 | 2.1216 | 3.7128 | 5.3040 |
**Prepared in 10mL volumetric flasks and made up to volume with acetonitrile.
(c): Spiking Solutions
This table provides a summary of information for spiking solutions. These include the volume and concentration of both the stock and the spiked solution, as well as the analysed concentrations for each of the following analytes: formaldehyde, acetone, butyraldehyde, and total butyraldehyde.
| Carbonyl | LFB Spiking Solution *** | |||||
|---|---|---|---|---|---|---|
| Stock [µg/mL] |
Volume (mL) |
Dilute to Vol. (mL) |
Spike [µg/mL] |
as Analyzed [µg/mL] |
||
| Formaldehyde | Primary | 225.879 | 1.4 | 31.62307 | 0.11294 | |
| Acetone | Primary | 303.064 | 1.0 | 10.0 | 30.30641 | 0.10824 |
| Butyraldehyde | Primary | 265.201 | 1.0 | 26.52008 | 0.09471 | |
| Total Butyraldehyde | 47.68601 | 0.1703 | ||||
***In a single 10mL volumetric flask and made up to volume with acetonitrile.
Figure 1: Analytical Chromatogram of Volatile Carbonyls in DNPH Extract of Mainstream Tobacco Smoke
The following figure is an analytical chromatogram of volatile carbonyls in DNPH Extract of Mainstream Tobacco Smoke.
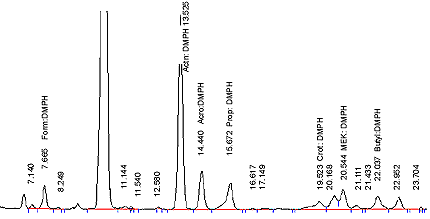
Figure 2: Chromatogram of a typical combined Carbonyl Calibration Standard
The following figure is a chromatogram of a typical combined carbonyl calibration standard.