Neutral Red Uptake Assay for Mainstream Tobacco Smoke
Health Canada
T-502, Second Edition
November 1, 2004
Table of Contents
- Scope of Applications
- Normative References
- Definitions and Abbrevations
- Method Summary
- Apparatus and Equipment
- Reagents and Supplies
- Preparation of Glassware and Plastic Ware
- Preparation of Solutions and Media
- Preparation of CHO Cell Culture Suspension
- Collection of Particulate Phase and Gas-Vapour Phase of the Smoke
- Preparation of Test Sample (PP; GVP; PP+GVP)
- The Cytotoxicity Test
- Calculation of Relative Absorbance
- Quality Control and Documentation
- Reporting of Assay Results
- References
- Appendices
1 Scope of Applications
- Applicable to the assessment of the cytotoxic potential of cigarette smoke. The method includes the preparation of Chinese Hamster Ovary (CHO) cells for assay, treatment with different fractions of cigarette smoke, uptake of neutral red dye by untreated and treated CHO cells and analysis of results.
- Applicable
to the assessment of:
- Particulate phase (PP) of cigarette smoke
- Gas-vapour phase (GVP) of cigarette smoke
- Combination of particulate phase and gas-vapour phase (PP+GVP).
2 Normative References
- Health Canada Official Method T-115. Determination of Tar, Water, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke. Second Edition. 2003. (Parts applicable to manufactured cigarettes only)
- International Organization for Standardization method ISO 3308. Cigarettes – Routine Analytical Cigarette-Smoking Machine - Definitions and Standard Conditions. 2000.
- International Organization for Standardization ISO 4387. Cigarettes – Determination of total and nicotine-free dry matter using a routine analytical smoking machine. 2000.
- US National Institutes of Health. NIH Publication No: 01-4500: Guidance Document on Using In Vitro Data to Estimate In Vivo Starting Doses for Acute Toxicity. 2001. (Appendix C only)
3 Definitions and Abbrevations
- CHO cells: Chinese hamster ovary cells.
- CMF-PBS:Phosphate-buffered saline without calcium chloride and magnesium chloride.
- Cytotoxicity: Cell injury, shedding of cells, death and/or impaired growth of a cell population caused by toxic substances.
- DMSO:Dimethylsulphoxide
- Glass fibre filter disc (pad): A pad used to collect particulate matter from tobacco smoke.
- GVP: Gas-vapour phase of the smoke.
- GVP+PP: A 1:1 (v/v) combination of gas-vapour phase and particulate phase.
- Hemocytometer (hemacytometer): A specialized microscopic grid used to count the number of cells in a suspension.
- IC50:Concentration that causes 50% reduction in cell activity as measured by absorbance of Neutral Red dye.
- Neutral Red:A dye that diffuses into viable cells and accumulates in lysosomes, where dye binds to anionic sites on the lysosomal matrix.
- PP:Particulate phase of the smoke.
- Replicate: A TPM preparation generated by an independent smoking of cigarettes taken from the same cigarette sample.
4 Method Summary
- The cigarettes (e.g. 20 cigarettes) are smoked under modified (intense) ISO conditions using a 20-port rotary smoking machine.
- The mainstream smoke is passed through a 92-mm glass fibre filter disc for PP collection, and into the cooled impinger containing phosphate-buffered saline (CMF-PBS) for GVP collection.
- Both fractions, either in DMSO (PP) or phosphate-buffered saline (GVP), are prepared to approximately 10 mg PP/mL solution (or 10 mg PP equivalent/mL solution in the case of GVP).
- Chinese
hamster ovary (CHO) cells are treated with the test sample in a tissue culture
plate within 1 hour of smoking, due to the instability of the GVP extract.
Following treatment, the test sample is removed and replaced with a neutral red
solution.
Note: Blank, negative (solvent) and positive controls are assayed concurrently. - Cells are washed and fixed, and the neutral red dye is then extracted using a glacial acetic acid/ethanol extraction solution.
- Absorbance is measured at 540 nm for each culture.
- Absorbance averages and standard deviations are calculated from 8 wells of the microtitre plate for each concentration of test sample. Cytotoxicity is indicated by a decreased ability of the injured cell to take up the neutral red dye into the lysosome. It is calculated by dividing the absorbance of the treated cells with the absorbance of the negative control cells multiplied by 100.
- The number of replicates is as per the applicable regulations.
Warning: The testing and evaluation of certain products against this test method may require the use of materials and or equipment that are potentially hazardous and this document does not purport to address all the safety aspects associated with its use. Anyone using this test method has the responsibility to consult with the appropriate authorities and to establish health and safety practices in conjunction with all existing applicable regulatory requirements prior to its use.
5 Apparatus and Equipment
- Equipment needed for collection of PP and GVP as per Appendix 1.
- All appropriate apparatus and equipment needed to perform the Neutral Red Uptake Assay. Refer to NIH Publication No:01-4500 (2001).
- Inverted microscope (or equivalent)
- Microplate reader (wavelength: 540nm) [e.g. Bio-Tek Instruments Inc. Universal Microplate Reader, model ELx800 or equivalent]
6 Reagents and Supplies
Note: Wherever possible, reagents are identified by their Chemical Abstract Service [CAS] registry numbers in square brackets. Use analytical grade chemicals whenever possible.
- Reagents
and supplies shall be as mentioned in NIH Publication No: 01-4500 (2001),
except as follows:
- CHO cells in growth media
- Neutral Red dye
- Fetal bovine serum
- Nutrient mixture (F-12 Ham with L-glutamine and NaHCO3)
- Calcium-magnesium free phosphate buffered saline (CMF-PBS)
- Penicillin-streptomycin solution
- Trypan blue solution (0.4%)
- Trypsin [9002-07-7 or equivalent]
- Formalin
- Sodium lauryl sulphate (SLS) [151-21-3]
- Ethanol (denatured, 2A) [64-17-5]
- 0.2 µM sterile filter
- Sterile deionised water
- Reagents and supplies needed for collection and preparation of test samples as per Appendix 1
7 Preparation of Glassware and Plastic Ware
- Glassware and plastic ware should be sterile, clean and, when necessary, disposable.
- Cleaning required for collection and preparation of test samples as per Appendix 1.
8 Preparation of Solutions and Media
Note: All reagent and media solutions preparation should follow standard aseptic procedures and whenever applicable follow the manufacturer instructions for the preparation of solutions.
Note: Potential carcinogen(s)/toxin(s) should be prepared in a fume hood with caution appropriate for this type of hazardous material.
- Prepare
the complete growth media by mixing 90% nutrient mixture with 10% fetal bovine
serum, 100 units/mL penicillin and 100 µg/mL streptomycin.
Note: Growth media is used as the diluent and the assay blank in this assay. - Prepare CMF-PBS following manufacturer’s instructions.
- Prepare 0.25% (w/v) trypsin solution in CMF-PBS one day prior to performing the assay.
- Prepare
a 50 µg/mL neutral red dye
solution (crystal-free) in nutrient mixture the day before required.
Place the solution, loosely capped, in a humidified CO2 incubator overnight at 37 ± 1°C and 5% CO2 atmosphere. The next day remove the crystals from the solution by filtration through sterile disposable 0.2 mm porosity filters.
Note: Alternatively, prepare 50 µg/mL neutral red dye solution (crystal-free) in nutrient mixture F-12 Ham with L-glutamine as per NIH Publication No: 01-4500 (2001). - Prepare 1% (v/v) formalin solution in sterile deionised water on day of use.
- Prepare a solution for extracting the neutral red dye containing: 50% (v/v) ethanol solution, 1% (v/v) glacial acetic acid, and 49% (v/v) sterile deionised water on day of use.
- Preparation of Negative Control
Solutions
- Prepare
a 2% (v/v) solution of each of the following solvents in growth media as the
negative controls:
- DMSO
- CMF-PBS
- DMSO/CMF-PBS (1:1)
- Prepare
a 2% (v/v) solution of each of the following solvents in growth media as the
negative controls:
- Preparation
of Positive Control Solutions
- Prepare a stock concentration of SLS solution in sterile deionised water (e.g. 20 mg SLS/mL).
- At the start
of an assay, prepare two appropriate concentrations of SLS solution in growth
media.
Note: One solution is estimated to be lethal to 50% of the cells (e.g. 110 µg/mL). One solution is estimated to be lethal to all cells (e.g. 200 µg/mL).
9 Preparation of CHO Cell Culture Suspension
- Calculate the amount of CHO cell suspension to harvest in order to conduct the assay. Twenty mL of a 50,000 viable cell/mL culture is required per 96 well assay plate. Set up and use four 96 well plates for each sample (8 wells per concentration per plate).
- CHO
cells are routinely grown as a monolayer in tissue culture grade flasks at 37 ±
1°C in a humidified atmosphere of 5% CO2. When cells approach
confluence they can be harvested or sub-cultured by trypsinization as follows:
- Remove the growth medium.
- Add CMF-PBS to rinse.
- Discard the washing solution.
- Add CMF-PBS for a second time to rinse.
- Discard the washing solution.
- Add 0.25% trypsin solution to the monolayer for desired period of time, and then remove solution.
- Add growth medium to the flask and mix well to make a single cell suspension.
- Counting the Number of CHO Cells in
Single Cell Suspension
- Mix 0.5 mL of cell suspension with 0.5 mL of 0.4% Trypan Blue solution. Care should be exercised when working with Trypan Blue, as it is toxic and may be carcinogenic.
- Allow this mixture to stand for at least 5 minutes but no more than 15 minutes.
- Make sure that hemocytometer is clean and dry prior to use. Keep the cover glass in place on the hemocytometer.
- Transfer the cell/trypan blue suspension to the chamber of hemocytometer with a capillary tube or other suitable device. Do not overfill or underfill the chambers.
- Count the number of viable and non-viable cells from 4 corner squares and 1 central square. Non-viable cells will stain blue.
- Calculate average viable cell count per square.
- Calculate
the number of viable cells per mL of cell suspension as follows:
Viable cells /mL = (Average cell count/square) × (Dilution factor) × 104 (Chamber conversion factor).
Example: (125 cells) × (2) × 104 = 2.5 × 106 cells/mL. - After determination of cell number, the cell suspension can be diluted with growth media and seeded into 96-well tissue culture plates at a density of 5 × 104 cells/mL or re-cultured into another flask.
10 Collection 0f Particulate Phase and Gas-vapour Phase of the Smoke
- Refer to Appendix 1: Collection and preparation of test samples.
11 Preparation of Test Sample (PP; GVP; PP+GVP)
- Refer to Appendix 1: Collection and preparation of test samples.
12 The Cytotoxicity Test
- Preparation of 96-Well Tissue Culture
Plate
- Leave the first column of wells free of CHO cells as the assay blank.
- Dispense 200 µL of the cell suspension into each of the remaining wells of 96-well tissue culture plate.
- Confirm presence or absence of CHO cells using an inverted microscope.
- Incubate the plate at 37 ± 1°C in a humidified, 5% CO2 atmosphere for 24 ± 3 hours.
- Preparing Desired Concentrations of
Sample and Controls for the Test
- Prepare the desired
concentrations of the negative, cigarette smoke sample (PP or GVP or PP+GVP) or
positive control solution by mixing with the appropriate amount of growth
medium.
Note: A range finder experiment as per NIH Publication No: 01-4500 (2001) may be required to ensure these concentrations produce approximately 10% to 90% inhibition of Neutral Red Uptake.
Note: It has been observed that for PP, GVP, and PP+GVP prepared from “typical” Canadian flue-cured cigarettes, concentrations of 0, 10, 50, 75, 100, 120, 140, 160, and 200 µg/mL will generally give a satisfactory response.
Note:Solvent used to prepare dilutions shall correspond to solvent used in preparation of test sample (e.g. DMSO, CMF-PBS, DMSO/CMF-PBS).
- Prepare the desired
concentrations of the negative, cigarette smoke sample (PP or GVP or PP+GVP) or
positive control solution by mixing with the appropriate amount of growth
medium.
- Exposing CHO Cells to Smoke Fractions
- Remove the culture medium from each well.
- Treat all 8 wells of each column (e.g. rows A-H) with either assay blank, negative control, positive control or each different concentration of the tobacco smoke fraction.
- Add 200 µL of growth medium containing negative control/positive control/differing concentrations of tobacco smoke fraction.
- Incubate the prepared 96-well plate at 37 ± 1°C in a humidified, 5% CO2 atmosphere for 24 hours.
- Treating CHO Cells with Neutral Red Dye
for Cellular Uptake
- Remove the medium from the wells after 24 hours incubation.
- Add 200 µL pre-warmed (37 ± 1°C) CMF-PBS.
- Remove CMF-PBS.
- Add 200 µL of freshly prepared filtered neutral red dye solution to each well.
- Incubate the cells at 37 ± 1°C for 3 hours in a humidified, 5% CO2 atmosphere.
- Fixing CHO Cells After Incubation with
1% Formalin
- Remove the neutral red solution from the plate.
- Add 200 µL of freshly prepared 1% formalin solution to each well.
- Remove the formalin solution after 1 minute but within 2 minutes of addition.
- Extraction of Neutral Red Dye from the
Fixed CHO Cells
- After removal of 1% Formalin solution from the wells, add 200 µL of freshly prepared 50% ethanol solution containing 1% acetic acid to each well.
- Shake the plate on a microtitre plate shaker for 5 - 10 minutes.
- Determination of Absorbance of Neutral
Red Dye Extracted from CHO Cells
- Read the absorbance from the wells containing the extracted neutral red solution on a microplate reader at a wavelength of 540 nm.
13 Calculation of Relative Absorbance
Note: In order to compare results between assays, the raw absorbance results from each assay plate are blank-corrected and normalized to the negative control prior to data analysis.
- The average absorbance of all assay blank wells is subtracted from each negative control, positive control and treatment well uncorrected absorbance value to produce blank-corrected absorbance values.
- To calculate relative absorbance, divide each blank-corrected positive control and treatment well absorbance value by the average blank-corrected negative control absorbance value for the plate. Express each absorbance fraction as a percent of the negative control to obtain the relative absorbance (%).
14 Quality Control and Documentation
- Chemicals and Media
- Verify and record the sterility of media, reagents and solutions as per good laboratory practice for tissue culture laboratories. Verify the performance characteristics of the control solutions.
- Cell Culture Maintenance
- The cell culture should be examined daily under an inverted microscope and any changes in morphology or adhesive properties noted.
- Cells should be checked regularly for the absence of mycoplasma contamination and only used if demonstrated to be mycoplasma-free (e.g. using Sigma mycoplasma staining kit).
- Laboratory Controls
- To assess the overall
performance of the analysis, a Kentucky Reference 2R4F control cigarette must
be included in the sample. (The results of the control cigarette may be
compared, using appropriate statistical techniques, to “expected values”
generated by the laboratory or, if none exist, to values found in literature.
This will provide information to the laboratory on test accuracy and
precision.)
Note: The IC50 result from each reference cigarette smoke fraction assay should be evaluated using appropriate control-charting techniques. For examples see Introduction to Statistical Quality Control, John Wiley & Sons, Inc., ISBN 0-471-51988-X. - Each 96-well tissue culture plate should contain a blank column that contains only media (to control for plate material absorbance).
- Each tissue culture plate should have one control column (8 rows) that contains solvent only (negative control).
- Each tissue culture plate should contain two 8-row columns containing differing concentrations of SLS, which function as positive controls.
- To assess the overall
performance of the analysis, a Kentucky Reference 2R4F control cigarette must
be included in the sample. (The results of the control cigarette may be
compared, using appropriate statistical techniques, to “expected values”
generated by the laboratory or, if none exist, to values found in literature.
This will provide information to the laboratory on test accuracy and
precision.)
- Evaluation of Negative Controls
- The average
uncorrected absorbance for the negative control of each assay plate should be
greater than the pre-defined minimum absorbance (see Table 1 below), which is
dependent on the solvent medium.
The following table displays the typical values for absorbance minimums. These include the uncorrected absorbance minimum taken at 540 nm for various smoke fraction and solvent control. Dimethylsulphoxide (DMSO) was used with particulate phase (PP) smoked fraction, obtaining an absorbance minimum of 0.3. Phosphate-buffered saline (PBS) was used with gas-vapour phase (GVP), obtaining a minimum of 0.2. Lastly, DMSO and PBS were used with PP and GVP, obtaining a minimum of 0.3.
Table 1: Typical Values for Absorbance Minimums Smoke Fraction
Solvent Control
Uncorrected Absorbance Minimum (540 nm)
PP
DMSO
0.3
GVP
PBS
0.2
PP + GVP
DMSO + PBS
0.3
- The average
uncorrected absorbance for the negative control of each assay plate should be
greater than the pre-defined minimum absorbance (see Table 1 below), which is
dependent on the solvent medium.
- Evaluation of Positive Controls
- The average relative absorbance of the positive controls must pass appropriate acceptance criteria defined by the individual laboratory.
15 Reporting of Assay Results
- Reports
of cytotoxicity data must include the following elements, as per Appendix 2:
- Sample ID (for reference to cigarette brand)
- Smoking data (smoking machine identity, smoking date, puff count, number of test samples smoked, total particulate matter, lag time)
- Uncorrected absorbance values for all assay blank, negative control, treatment and positive control wells of each assay plate (include average, standard deviation and coefficient of variation statistics for each assay plate column)
- Blank-corrected absorbance values for all negative control, treatment and positive control wells of each assay plate (include average, standard deviation and coefficient of variation statistics for each assay plate column)
- Relative absorbance values for all treatment and positive control wells of each assay plate (include average, standard deviation and coefficient of variation statistics for each assay plate column)
- Data for PP alone, GVP alone, and combination PP+GVP
16 References
- Babich, H. and Borenfreund, E. (1990) Cytotoxic effects of food additives and pharmaceuticals on cells in culture as determined with the Neutral Red assay. Journal of Pharmaceutical Sciences 79: 592-594.
- Bombick, B.R., Murli, H., Avalos, J.T., Bombick, D.W., Morgan, W.T., Putnam, K.P., and Doolittle, D.J. (1997) Chemical and biological studies of a new cigarette that primarily heats tobacco. Part 2. In vitro toxicology of mainstream smoke condensate. Food and Chemical Toxicology 36: 183-190.
- Bombick, D.W., Ayres, P.H., Putnam, K., Bombick, B.R., and Doolittle, D.J. (1998) Chemical and biological studies of a new cigarette that primarily heats tobacco. Part 3. In vitro toxicity of whole smoke. Food and Chemical Toxicology 36: 191-197.
- Borenfreund, E. and Puerner, J.A. (1985) Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicology Letters 24: 119-124.
Appendices
Appendix 1
Preparation and Collection of Test Samples for Neutral Red Cytotoxicity Assay
1 Summary
- The cigarettes (e.g. 20 cigarettes) are smoked under modified (intense) ISO conditions using a 20-port rotary smoking machine.
- Mainstream smoke is passed through a 92-mm glass fibre filter disc for PP collection, and into the cooled impinger containing phosphate-buffered saline (CMF-PBS) for GVP collection.
- Both
fractions, either in DMSO (PP) or phosphate-buffered saline (GVP), are prepared
to approximately 10 mg PP/mL solution (or 10 mg PP equivalent/mL solution in
the case of GVP).
Note:Smoke a sufficient amount of cigarettes such that breakthrough of particulate matter does not occur, and the limits of total particulate matter, defined in ISO 4387, are not exceeded. The number of cigarettes will also need to be adjusted to provide a minimum of 180 mg total particulate matter per 92-mm disc collection.
2 Apparatus and Equipment
- Equipment needed to perform conditioning as specified in Health Canada Official Method T-115.
- Equipment needed to perform marking for butt length as specified in Health Canada Official Method T-115.
- Equipment needed to perform smoking of tobacco product as specified in Health Canada Official Method T-115.
3 Reagents and Supplies
Note: Wherever possible, reagents are identified by their Chemical Abstract Service [CAS] registry numbers in square brackets. All reagents shall be at least analytical grade.
- Dimethylsulphoxide (DMSO) [67-68-5]
- Sterile phosphate buffered Saline (without CaCl2 and MgCl2) (CMF-PBS)
- Ethanol [67-17-5]
- Sterile serological pipettes (5 mL and 10 mL)
- Impinger (approx. volume 70 mL) with extra coarse (EC) frit (Kimble 170-200 µm pore diameter) and Teflon sleeve
- Glass fibre filter disc (e.g. Cambridge filter pad or equivalent) and holder
- Ice water bath and thermometer
- Eppendorf (or equivalent) pipettes and sterile tips (various sizes)
- Sterile conical tubes (15 mL and 50 mL)
- Sterile cheese cloth
- Aluminum foil
- Tubing and connectors (Nalgene food grade or equivalent)
- Polymethylpentene (PMP) Erlenmeyer flask (125 mL) or equivalent
4 Preparation of Glassware
- Clean and dry glassware in such a manner to ensure that contamination does not occur.
- All lab ware to be used should be sterilized by autoclaving at 121oC for 30 minutes at 15 pounds per square inch (psi).
5 Sampling
- The sampling of cigarettes for the purpose of testing shall be as specified in Health Canada Official Method T-115.
6 Cigarette Preparation
- Mark test samples for butt length as specified in Health Canada Official Method T-115.
- Prepare cigarettes to be smoked as specified in Health Canada Official Method T-115.
- Condition cigarettes as specified in Health Canada Official Method T-115.
7 Preparation Of Impingers
Note: All procedures are to be performed such that background contamination is minimized by sterilizing/disinfecting equipment and work surfaces.
- Wrap the following separately in aluminum foil and autoclave: impinger base, regular impinger stem with EC frit, top and side connectors.
- Cut the plastic tubing into 30 cm (12 inch) and 20 cm (8 inch) lengths.
- Rinse the inside of the tubing as well as the plastic tube connectors with ethanol in order to disinfect and wrap in aluminum foil.
- Assemble
the tubing and plastic connectors on the impinger stem with the EC frit. See
Figure 1 for proper assembly.
Figure 1: Preparation of Impingers for Gas-vapour Phase Collection
The following figure shows the preparation of impinger for a gas-vapour phase collection.
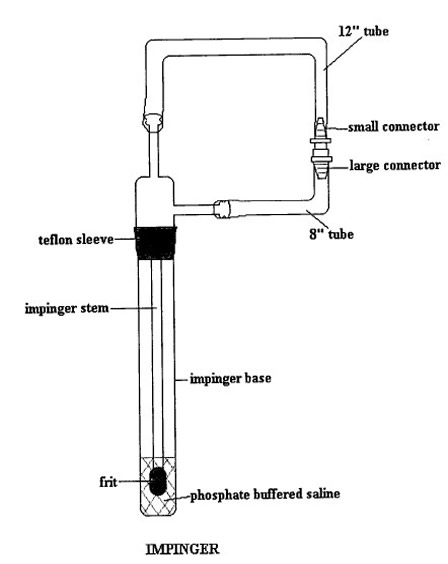
Note: Do not remove the aluminum foil wrapping from around the regular stem EC frit and sleeve. Make sure the Teflon sleeve has no creases or holes. - Add 15mL of ice cold CMF-PBS to impinger base using serological pipette.
- Gently remove the aluminum foil wrapped around the regular stem with EC frit and sleeve, immerse the stem into the CMF-PBS.
- Temporarily remove foil wrapping around base to ensure that the EC frit is immersed in the CMF-PBS. Replace the foil.
- Place the closed impinger unit into ice bath containing ice, water and thermometer ensuring that the temperature does not exceed 1oC.
8 Smoking Machine Preparation
- The ambient conditions for smoking shall be as specified in Health Canada Official Method T-115.
- Use only non-UV lighting in the rooms in which the sample generation and sample analyses are conducted.
- The
machine conditions for a rotary machine shall be as specified in Health Canada
Official Method T-115 noting the following:
- To reduce bacterial contamination, all neoprene washers and labyrinth seals are to be cleaned with 70% ethanol.
- To
reduce bacterial contamination, various smoking machine parts are cleaned with
a 70% ethanol solution prior to smoking. These parts are as follows:
- Ash plate
- Ports
- Pad holders
- Smoke train
- All work surfaces, including the exterior of extraction flasks and stoppers
- Any other items, such as gloves worn by the technician that may come in contact with the sample or cleaned work surfaces
- Assemble
the smoke train as illustrated in Figure 2.
Figure 2: Sample Collection of Particulate and Gas Phase Samples for NRU Assay
The following figure shows the sample collection of particulate and gas phase samples for Neutral Red Uptake (NRU) Assay.
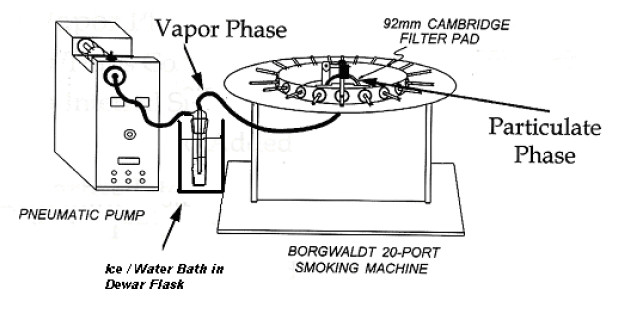
9 Sample Generation
- Smoke
the cigarettes using the above smoke train and collect the total particulate
matter as specified in Health Canada Official method T-115 with the following
modifications:
- The smoking conditions are
modified in the following manner:
- puff volume is increased from 35 mL to 55 mL,
- puff interval is decreased from 60 s to 30 s, and
- all ventilation holes are blocked by placing over them a strip of Mylar adhesive tape, Scotch Brand product no. 600 Transparent Tape, and the tape must be cut so that it covers the circumference and is tightly secured from the end of the filter to the tipping over-wrap seam, or by another method of equivalent efficiency.
- After smoking the required number of test samples, perform three clearing puffs.
- Record the time at which smoking is complete as well as sample preparation/processing time.
- Record the temperature of the ice bath in which impinger is kept.
- Disassemble smoke train by removing the pad holder from the smoking machine and close the impinger by connecting the two ends together.
- The smoking conditions are
modified in the following manner:
- The number of replicates to be generated is as per applicable regulations. All replicates must be analyzed on the same day.
- Determine the total particulate matter as specified in Health Canada Official Method T-115.
10 Sample Preparation
- Particulate Phase
- Upon completion of total particulate matter determination, the pad is to be removed from the pad holder, folded into quarters (total particulate matter side in), and the pad holder wiped with the folded pad.
- Transfer the collection pad to a sterile 125 mL PMP Erlenmeyer flask.
- Pipette the appropriate amount of DMSO to the flask such that the final concentration of total particulate matter is 10 mg/mL.
- Record the amount of DMSO added to the flask.
- Extract the pad in the PMP Erlenmeyer flask for 20 minutes on the wrist action shaker
- This solution is then filtered through sterile cheesecloth and administered to the cells within approximately 1 hour after the completion of sample generation.
- Gas-Vapour Phase
- Upon completion of total particulate matter determination, transfer additional cold CMF-PBS to the impinger, such that the total volume of CMF-PBS is equal to the amount of DMSO used to extract the particulate phase.
- Record the total amount of CMF-PBS added to the flask such that the GVP concentration is representative of a 10 mg PP equivalent/mL CMF-PBS.
- Ensure that CMF-PBS in the impinger is mixed properly.
- Transfer enough impinger solution to carry out testing to a 50 mL sterile conical tube.
- Cover the tubes with clean aluminum foil.
- The CMF-PBS sample (as per Section 10.2.4) solution is to be administered to the cells within 1 hour of the completion of sample generation.
- Particulate Phase and Gas-Vapour Phase –
Combined
- Pipette the appropriate amount of impinger solution (e.g. 1.5 mL) to a 15 mL sterile conical tube.
- Pipette an equal amount (e.g. 1.5 mL) of the particulate phase extract to the same 15 mL sterile conical tube that contains the CMF-PBS aliquot, such that the combined solution contains equal amounts of total particulate matter (or PP equivalent) from the particulate phase and gas-vapour phase.
- Ensure the sample is adequately mixed before being administered to the cells.
Appendix 2
Sample Reporting Formats For NRU Assay Data
1. Sample ID
The following table provides description for lab sample IDs, including 030001, 030002,030003.
| Laboratory Sample ID | Sample Description |
|---|---|
| 030001 | Kentucky Reference 2R4F |
| 030002 | Brand X regular full flavour |
| 030003 | Brand Y King size Medium |
2. Smoking Data
The following table includes the smoking data for various runs performed on set 1. Four samples (030001, 030002, and 030003) were tested with 3 replicates each. The smoking date was on either February 10 or February 18 of 2004. 11, 20 or 100 cigarettes were smoked, and a rotary smoking machine was used. Between 7.1 to 936 puffs were used per cigarette. The weight of the mainstream total particulate matter and the lag time were recorded.
| Set Number | Run Number | Sample ID | Smoke Fraction | Replicate Number | Smoking Date | Cigarettes Smoked | Puff Count(per cig.) | Weight of MSTPM(mg) 1 | Smoking Machine | Lag Time 2(hours) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 030001 | A | 1 | February 10, 2004 | 20 | 9.6 | 218 | Borgwaldt rotary | 00:59 |
| 2 | 1 | 030001 | B | 1 | February 18, 2004 | 20 | 9.2 | 243 | Borgwaldt rotary | 00:19 |
| 2 | 1 | 030001 | C | 1 | February 18, 2004 | 20 | 9.2 | 243 | Borgwaldt rotary | 00:46 |
| 1 | 3 | 030002 | A | 1 | February 10, 2004 | 100 | 7.1 | 203 | Borgwaldt rotary | 00:51 |
| 1 | 3 | 030002 | B | 1 | February 10, 2004 | 100 | 7.1 | 203 | Borgwaldt rotary | 00:19 |
| 1 | 3 | 030002 | C | 1 | February 10, 2004 | 100 | 7.1 | 203 | Borgwaldt rotary | 00:39 |
| 2 | 2 | 030003 | A | 1 | February 18, 2004 | 11 | 7.5 | 219 | Borgwaldt rotary | 00:54 |
| 1 | 2 | 030003 | B | 1 | February 10, 2004 | 11 | 7.6 | 215 | Borgwaldt rotary | 00:17 |
| 2 | 2 | 030003 | C | 1 | February 18, 2004 | 11 | 7.5 | 219 | Borgwaldt rotary | 00:47 |
Note: The following codes are used to indicate the applied fraction of mainstream smoke:
A - Particulate (DMSO), B - Gas/Vapour (PBS), C - A + B (DMSO + PBS)
- Samples extracted in appropriate solvent control to give a final concentration of 10.0 mg/mL
- Time lapsed between completion of smoking and test initiation (in hours)
3. Uncorrected Absorbance Data
The following table displays uncorrected absorbance data for neutral red cytotoxicity assay results. This includes the raw assay plate absorbance ratings for each sample. The properties of the sample ranges from sample ID, smoke fraction, replicate number, plate number and well number. The assay blank and control readings are recorded, as well as the absorbance readings for various cigarette smoke condensate concentrations (ranging from 10 to 200 ug/mL), as well as for various sodium lauryl sulphate (SLS) concentrations (ranging from 110 to 200 ug/mL).
| Set-Run Number | Sample ID | Smoke Fraction | Replicate Number | Plate Number | Well Number | Raw Assay Plate Absorbance Readings | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay Blank | Control *Reading | Cigarette Smoke Condensate (µg/mL) | SLS (µg/mL) | ||||||||||||||
| 10 | 50 | 75 | 100 | 120 | 140 | 160 | 200 | 110 | 200 | ||||||||
| 1-1 | 030001 | A | 1 | 1 | 1 | 0.082 | 0.479 | 0.495 | 0.424 | 0.316 | 0.204 | 0.136 | 0.118 | 0.101 | 0.09 | 0.139 | 0.085 |
| 1-1 | 030001 | A | 1 | 1 | 2 | 0.085 | 0.505 | 0.523 | 0.402 | 0.353 | 0.207 | 0.147 | 0.123 | 0.105 | 0.093 | 0.116 | 0.086 |
| 1-1 | 030001 | A | 1 | 1 | 3 | 0.084 | 0.508 | 0.488 | 0.401 | 0.32 | 0.23 | 0.116 | 0.113 | 0.096 | 0.086 | 0.109 | 0.083 |
| 1-1 | 030001 | A | 1 | 1 | 4 | 0.082 | 0.471 | 0.473 | 0.418 | 0.24 | 0.211 | 0.114 | 0.104 | 0.096 | 0.086 | 0.104 | 0.084 |
| 1-1 | 030001 | A | 1 | 1 | 5 | 0.083 | 0.507 | 0.538 | 0.404 | 0.348 | 0.218 | 0.119 | 0.107 | 0.094 | 0.088 | 0.125 | 0.085 |
| 1-1 | 030001 | A | 1 | 1 | 6 | 0.084 | 0.499 | 0.48 | 0.396 | 0.28 | 0.193 | 0.115 | 0.1 | 0.094 | 0.089 | 0.116 | 0.103 |
| 1-1 | 030001 | A | 1 | 1 | 7 | 0.082 | 0.505 | 0.514 | 0.403 | 0.322 | 0.199 | 0.116 | 0.102 | 0.09 | 0.085 | 0.118 | 0.09 |
| 1-1 | 030001 | A | 1 | 1 | 8 | 0.08 | 0.477 | 0.474 | 0.387 | 0.277 | 0.183 | 0.118 | 0.098 | 0.097 | 0.084 | 0.129 | 0.086 |
| Average | 0.083 | 0.494 | 0.498 | 0.404 | 0.307 | 0.206 | 0.123 | 0.108 | 0.097 | 0.088 | 0.120 | 0.088 | |||||
| Std. Dev. | 0.002 | 0.015 | 0.024 | 0.012 | 0.039 | 0.015 | 0.012 | 0.009 | 0.005 | 0.003 | 0.011 | 0.006 | |||||
| Coeff. Var. | 1.9 | 3.1 | 4.9 | 2.9 | 12.5 | 7.1 | 9.9 | 8.3 | 4.8 | 3.4 | 9.4 | 7.4 | |||||
| 1-1 | 030001 | A | 1 | 2 | 1 | 0.08 | 0.482 | 0.47 | 0.389 | 0.314 | 0.227 | 0.13 | 0.117 | 0.102 | 0.089 | 0.103 | 0.085 |
| 1-1 | 030001 | A | 1 | 2 | 2 | 0.08 | 0.498 | 0.506 | 0.394 | 0.303 | 0.227 | 0.13 | 0.113 | 0.104 | 0.096 | 0.103 | 0.085 |
| 1-1 | 030001 | A | 1 | 2 | 3 | 0.082 | 0.494 | 0.527 | 0.41 | 0.31 | 0.191 | 0.123 | 0.106 | 0.096 | 0.088 | 0.11 | 0.084 |
| 1-1 | 030001 | A | 1 | 2 | 4 | 0.081 | 0.513 | 0.512 | 0.415 | 0.311 | 0.205 | 0.118 | 0.097 | 0.096 | 0.087 | 0.107 | 0.084 |
| 1-1 | 030001 | A | 1 | 2 | 5 | 0.081 | 0.505 | 0.503 | 0.396 | 0.306 | 0.192 | 0.101 | 0.099 | 0.086 | 0.085 | 0.107 | 0.085 |
| 1-1 | 030001 | A | 1 | 2 | 6 | 0.081 | 0.487 | 0.538 | 0.438 | 0.363 | 0.189 | 0.103 | 0.1 | 0.095 | 0.087 | 0.107 | 0.083 |
| 1-1 | 030001 | A | 1 | 2 | 7 | 0.082 | 0.494 | 0.516 | 0.405 | 0.351 | 0.196 | 0.115 | 0.094 | 0.091 | 0.085 | 0.107 | 0.084 |
| 1-1 | 030001 | A | 1 | 2 | 8 | 0.079 | 0.506 | 0.51 | 0.379 | 0.326 | 0.187 | 0.12 | 0.098 | 0.097 | 0.086 | 0.099 | 0.084 |
| Average | 0.081 | 0.497 | 0.510 | 0.403 | 0.323 | 0.202 | 0.118 | 0.103 | 0.096 | 0.088 | 0.105 | 0.084 | |||||
| Std. Dev. | 0.001 | 0.010 | 0.020 | 0.018 | 0.022 | 0.017 | 0.011 | 0.008 | 0.006 | 0.004 | 0.003 | 0.001 | |||||
| Coeff. Var. | 1.3 | 2.1 | 3.9 | 4.5 | 6.9 | 8.2 | 9.3 | 8.0 | 5.9 | 4.1 | 3.3 | 0.8 | |||||
Note: The following codes are used to indicate the applied fraction of mainstream smoke:
A - Particulate (DMSO), B - Gas/Vapour (PBS), C - A + B (DMSO + PBS)
4. Blank-corrected Absorbance Data
The following table displays blank-corrected absorbance data for neutral red cytotoxicity assay results. This includes the raw assay plate absorbance ratings for each sample. The properties of the sample ranges from sample ID, smoke fraction, replicate number, plate number and well number. The assay blank and control readings are recorded, as well as the absorbance readings for various cigarette smoke condensate concentrations (ranging from 10 to 200 ug/mL), as well as for various SLS concentrations (ranging from 110 to 200 ug/mL).
| Run-Port Number | Sample ID | Smoke Fraction | Replicate Number | Plate Number | Well Number | Blank-Corrected Assay Plate Absorbance Readings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control * Reading | Cigarette Smoke Condensate (µg/mL) | SLS (µg/mL) | ||||||||||||||
| 10 | 50 | 75 | 100 | 120 | 140 | 160 | 200 | 110 | 200 | |||||||
| 1-1 | 030001 | A | 1 | 1 | 1 | 0.396 | 0.412 | 0.341 | 0.233 | 0.121 | 0.053 | 0.035 | 0.018 | 0.007 | 0.056 | 0.002 |
| 1-1 | 030001 | A | 1 | 1 | 2 | 0.422 | 0.440 | 0.319 | 0.270 | 0.124 | 0.064 | 0.040 | 0.022 | 0.010 | 0.033 | 0.003 |
| 1-1 | 030001 | A | 1 | 1 | 3 | 0.425 | 0.405 | 0.318 | 0.237 | 0.147 | 0.033 | 0.030 | 0.013 | 0.003 | 0.026 | 0.000 |
| 1-1 | 030001 | A | 1 | 1 | 4 | 0.388 | 0.390 | 0.335 | 0.157 | 0.128 | 0.031 | 0.021 | 0.013 | 0.003 | 0.021 | 0.001 |
| 1-1 | 030001 | A | 1 | 1 | 5 | 0.424 | 0.455 | 0.321 | 0.265 | 0.135 | 0.036 | 0.024 | 0.011 | 0.005 | 0.042 | 0.002 |
| 1-1 | 030001 | A | 1 | 1 | 6 | 0.416 | 0.397 | 0.313 | 0.197 | 0.110 | 0.032 | 0.017 | 0.011 | 0.006 | 0.033 | 0.020 |
| 1-1 | 030001 | A | 1 | 1 | 7 | 0.422 | 0.431 | 0.320 | 0.239 | 0.116 | 0.033 | 0.019 | 0.007 | 0.002 | 0.035 | 0.007 |
| 1-1 | 030001 | A | 1 | 1 | 8 | 0.394 | 0.391 | 0.304 | 0.194 | 0.100 | 0.035 | 0.015 | 0.014 | 0.001 | 0.046 | 0.003 |
| Average | 0.411 | 0.415 | 0.322 | 0.224 | 0.123 | 0.040 | 0.025 | 0.014 | 0.005 | 0.037 | 0.005 | |||||
| Std. Dev. | 0.015 | 0.024 | 0.012 | 0.039 | 0.015 | 0.012 | 0.009 | 0.005 | 0.003 | 0.011 | 0.006 | |||||
| Coeff. Var. | 3.8 | 5.8 | 3.6 | 17.2 | 11.9 | 30.4 | 35.5 | 33.1 | 61.0 | 30.5 | 129.9 | |||||
| 1-1 | 030001 | A | 1 | 2 | 1 | 0.401 | 0.389 | 0.308 | 0.233 | 0.146 | 0.049 | 0.036 | 0.021 | 0.008 | 0.022 | 0.004 |
| 1-1 | 030001 | A | 1 | 2 | 2 | 0.417 | 0.425 | 0.313 | 0.222 | 0.146 | 0.049 | 0.032 | 0.023 | 0.015 | 0.022 | 0.004 |
| 1-1 | 030001 | A | 1 | 2 | 3 | 0.413 | 0.446 | 0.329 | 0.229 | 0.110 | 0.042 | 0.025 | 0.015 | 0.007 | 0.029 | 0.003 |
| 1-1 | 030001 | A | 1 | 2 | 4 | 0.432 | 0.431 | 0.334 | 0.230 | 0.124 | 0.037 | 0.016 | 0.015 | 0.006 | 0.026 | 0.003 |
| 1-1 | 030001 | A | 1 | 2 | 5 | 0.424 | 0.422 | 0.315 | 0.225 | 0.111 | 0.020 | 0.018 | 0.005 | 0.004 | 0.026 | 0.004 |
| 1-1 | 030001 | A | 1 | 2 | 6 | 0.406 | 0.457 | 0.357 | 0.282 | 0.108 | 0.022 | 0.019 | 0.014 | 0.006 | 0.026 | 0.002 |
| 1-1 | 030001 | A | 1 | 2 | 7 | 0.413 | 0.435 | 0.324 | 0.270 | 0.115 | 0.034 | 0.013 | 0.010 | 0.004 | 0.026 | 0.003 |
| 1-1 | 030001 | A | 1 | 2 | 8 | 0.425 | 0.429 | 0.298 | 0.245 | 0.106 | 0.039 | 0.017 | 0.016 | 0.005 | 0.018 | 0.003 |
| Average | 0.417 | 0.430 | 0.323 | 0.242 | 0.121 | 0.037 | 0.022 | 0.015 | 0.007 | 0.025 | 0.004 | |||||
| Std. Dev. | 0.010 | 0.020 | 0.018 | 0.022 | 0.017 | 0.011 | 0.008 | 0.006 | 0.004 | 0.003 | 0.001 | |||||
| Coeff. Var. | 2.5 | 4.6 | 5.6 | 9.2 | 13.6 | 29.8 | 36.9 | 37.6 | 50.0 | 14.1 | 20.2 | |||||
Note: The following codes are used to indicate the applied fraction of mainstream smoke:
A - Particulate (DMSO), B - Gas/Vapour (PBS), C - A + B (DMSO + PBS)
5. Relative Absorbance Data
The following table displays the relative absorbance data for neutral red cytotoxicity assay results. This includes the raw assay plate absorbance ratings for each sample. The properties of the sample ranges from sample ID, smoke fraction, replicate number, plate number and well number. The assay blank and control readings are recorded, as well as the absorbance readings for various cigarette smoke condensate concentrations (ranging from 10 to 200 ug/mL), as well as for various SLS concentrations (ranging from 110 to 200 ug/mL).
| Run-Port Number | Sample ID | Smoke Fraction | Replicate Number | Plate Number | Well Number | Relative Assay Plate Absorbance Readings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cigarette Smoke Condensate (µg/mL) | SLS (µg/mL) | ||||||||||||||
| 10 | 50 | 75 | 100 | 120 | 140 | 160 | 200 | 110 | 200 | ||||||
| 1-1 | 030001 | A | 1 | 1 | 1 | 100 | 83.0 | 56.7 | 29.5 | 13.0 | 8.57 | 4.44 | 1.76 | 13.7 | 0.547 |
| 1-1 | 030001 | A | 1 | 1 | 2 | 107 | 77.7 | 65.7 | 30.2 | 15.6 | 9.79 | 5.41 | 2.49 | 8.09 | 0.791 |
| 1-1 | 030001 | A | 1 | 1 | 3 | 98.6 | 77.4 | 57.7 | 35.8 | 8.09 | 7.36 | 3.22 | 0.791 | 6.38 | 0.061 |
| 1-1 | 030001 | A | 1 | 1 | 4 | 94.9 | 81.5 | 38.2 | 31.2 | 7.60 | 5.17 | 3.22 | 0.791 | 5.17 | 0.304 |
| 1-1 | 030001 | A | 1 | 1 | 5 | 111 | 78.1 | 64.5 | 32.9 | 8.82 | 5.90 | 2.74 | 1.28 | 10.3 | 0.547 |
| 1-1 | 030001 | A | 1 | 1 | 6 | 96.6 | 76.2 | 48.0 | 26.8 | 7.84 | 4.20 | 2.74 | 1.52 | 8.09 | 4.93 |
| 1-1 | 030001 | A | 1 | 1 | 7 | 105 | 77.9 | 58.2 | 28.3 | 8.09 | 4.68 | 1.76 | 0.547 | 8.57 | 1.76 |
| 1-1 | 030001 | A | 1 | 1 | 8 | 95.2 | 74.0 | 47.2 | 24.4 | 8.57 | 3.71 | 3.47 | 0.304 | 11.2 | 0.791 |
| Average | 101 | 78.2 | 54.5 | 29.9 | 9.70 | 6.17 | 3.37 | 1.19 | 8.94 | 1.22 | |||||
| Std. Dev. | 6 | 2.9 | 9.4 | 3.6 | 2.95 | 2.19 | 1.12 | 0.72 | 2.72 | 1.58 | |||||
| Coeff. Var. | 5.8 | 3.6 | 17.2 | 11.9 | 30.4 | 35.5 | 33.1 | 61.0 | 30.5 | 129.9 | |||||
| 1-1 | 030001 | A | 1 | 2 | 1 | 93.4 | 74.0 | 56.0 | 35.1 | 11.8 | 8.70 | 5.10 | 1.98 | 5.34 | 1.02 |
| 1-1 | 030001 | A | 1 | 2 | 2 | 102 | 75.2 | 53.3 | 35.1 | 11.8 | 7.74 | 5.58 | 3.66 | 5.34 | 1.02 |
| 1-1 | 030001 | A | 1 | 2 | 3 | 107 | 79.0 | 55.0 | 26.5 | 10.1 | 6.06 | 3.66 | 1.74 | 7.02 | 0.780 |
| 1-1 | 030001 | A | 1 | 2 | 4 | 104 | 80.2 | 55.3 | 29.8 | 8.94 | 3.90 | 3.66 | 1.50 | 6.30 | 0.780 |
| 1-1 | 030001 | A | 1 | 2 | 5 | 101 | 75.7 | 54.1 | 26.7 | 4.86 | 4.38 | 1.26 | 1.02 | 6.30 | 1.02 |
| 1-1 | 030001 | A | 1 | 2 | 6 | 110 | 85.7 | 67.7 | 26.0 | 5.34 | 4.62 | 3.42 | 1.50 | 6.30 | 0.540 |
| 1-1 | 030001 | A | 1 | 2 | 7 | 104 | 77.8 | 64.9 | 27.7 | 8.22 | 3.18 | 2.46 | 1.02 | 6.30 | 0.780 |
| 1-1 | 030001 | A | 1 | 2 | 8 | 103 | 71.6 | 58.9 | 25.5 | 9.42 | 4.14 | 3.90 | 1.26 | 4.38 | 0.780 |
| Average | 103 | 77.4 | 58.1 | 29.0 | 8.82 | 5.34 | 3.63 | 1.71 | 5.91 | 0.840 | |||||
| Std. Dev. | 5 | 4.4 | 5.3 | 4.0 | 2.63 | 1.97 | 1.37 | 0.86 | 0.83 | 0.170 | |||||
| Coeff. Var. | 4.6 | 5.6 | 9.2 | 13.6 | 29.8 | 36.9 | 37.6 | 50.0 | 14.1 | 20.2 | |||||
Note: The following codes are used to indicate the applied fraction of mainstream smoke:
A - Particulate (DMSO), B - Gas/Vapour (PBS), C - A + B (DMSO + PBS)